Abstract
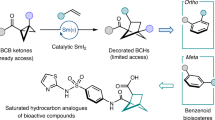
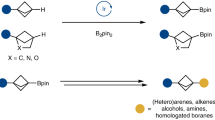
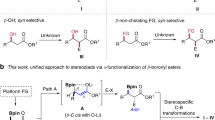
Bicyclic hydrocarbons, and bicyclo[1.1.1]pentanes (BCPs) in particular, are playing an emerging role as saturated bioisosteres in pharmaceutical, agrochemical and materials chemistry. Taking advantage of strain-release strategies, prior synthetic studies have featured the synthesis of bridgehead-substituted (C1, C3) BCPs from [1.1.1]propellane. Here, we describe an approach to access multisubstituted BCPs via intramolecular cyclization. In addition to C1,C3-disubstituted BCPs, this method also enables the construction of underexplored multisubstituted (C1, C2 and C3) BCPs from readily accessible cyclobutanones. The broad generality of this method has also been examined through the synthesis of a variety of other caged bicyclic molecules, ranging from [2.1.1] to [3.2.1] scaffolds. The modularity afforded by the pendant bridgehead boron pinacol esters generated during the cyclization reaction has been demonstrated through several downstream functionalizations, highlighting the ability of this approach to enable the programmed and divergent synthesis of multisubstituted bicyclic hydrocarbons.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
The experimental data as well as the characterization data for all the compounds prepared in the course of these studies are provided in the Supplementary Information. The crystallographic data for the structures reported in this article have been deposited at the Cambridge Crystallographic Data Centre, under deposition numbers CCDC 2062923 (16), 2062924 (20), 2062925 (26) and 2081087 (40-ester; see the X-ray crystallographic data in the Supplementary Information). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/.
References
Levin, M. D., Kaszynski, P. & Michl, J. Bicyclo[1.1.1]pentanes, [n]staffanes, [1.1.1]propellanes, and tricyclo[2.1.0.02,5]pentanes. Chem. Rev. 100, 169–234 (2000).
Dilmaç, A. M., Spuling, E., de Meijere, A. & Bräse, S. Propellanes—from a chemical curiosity to ‘explosive’ materials and natural products. Angew. Chem. Int. Ed. 56, 5684–5718 (2017).
Wiberg, K. B. The concept of strain in organic chemistry. Angew. Chem. Int. Ed. Engl. 25, 312–322 (1986).
Lovering, F., Bikker, J. & Humblet, C. Escape from flatland: increasing saturation as an approach to improving clinical success. J. Med. Chem. 52, 6752–6756 (2009).
Mikhailiuk, P. K. et al. Conformationally rigid trifluoromethyl‐substituted α‐amino acid designed for peptide structure analysis by solid‐state 19F NMR spectroscopy. Angew. Chem. Int. Ed. 45, 5659–5661 (2006).
Stepan, A. F. et al. Application of the bicyclo[1.1.1]pentane motif as a nonclassical phenyl ring bioisostere in the design of a potent and orally active γ-secretase inhibitor. J. Med. Chem. 55, 3414–3424 (2012).
Westphal, M. V., Wolfstaedter, B. T., Plancher, J., Gatfield, J. & Carreira, E. M. Evaluation of tert-butyl isosteres: case studies of physicochemical and pharmacokinetic properties, efficacies, and activities. ChemMedChem 10, 461–469 (2015).
Costantino, G. et al. Synthesis and biological evaluation of 2-(3′-(1H-tetrazol-5-yl)bicyclo[1.1.1]pent-1-yl)glycine (S-TBPG), a novel mGlu1 receptor antagonist. Bioorg. Med. Chem. 9, 221–227 (2001).
Measom, N. D. et al. Investigation of a bicyclo[1.1.1]pentane as a phenyl replacement within an LpPLA2 inhibitor. ACS Med. Chem. Lett. 8, 43–48 (2017).
Auberson, Y. P. et al. Improving nonspecific binding and solubility: bicycloalkyl groups and cubanes as para-phenyl bioisosteres. ChemMedChem 12, 590–598 (2017).
Talele, T. T. Opportunities for tapping into three-dimensional chemical space through a quaternary carbon. J. Med. Chem. 63, 13291–13315 (2020).
Bauer, M. R. et al. Put a ring on it: application of small aliphatic rings in medicinal chemistry. RSC Med. Chem. 12, 448–471 (2021).
Mikhailiuk, P. K. Saturated bioisoteres of benzene: where to go next? Org. Biomol. Chem. 17, 2839–2849 (2019).
Denisenko, A., Garbuz, P., Shishkina, S. V., Voloshchuk, N. M. & Mikhailiuk, P. K. Saturated bioisosteres of ortho-substituted benzenes. Angew. Chem. Int. Ed. 59, 20515–20521 (2020).
Wiberg, K. B. & Walker, F. H. [1.1.1]Propellane. J. Am. Chem. Soc. 104, 5239–5240 (1982).
Jackson, J. E. & Allen, L. C. The C1–C3 bond in [1.1.1]propellane. J. Am. Chem. Soc. 106, 591–599 (1984).
Feller, D. & Davidson, E. R. Ab initio studies of [1.1.1]- and [2.2.2]propellane. J. Am. Chem. Soc. 109, 4133–4139 (1987).
Wu, W., Gu, J., Song, J., Shaik, S. & Hiberty, P. C. The inverted bond in [1.1.1]propellane is a charge-shift bond. Angew. Chem. Int. Ed. 48, 1407–1410 (2009).
Wiberg, K. B., Waddell, S. T. & Laidig, K. [1.1.1]Propellane: reaction with free radicals. Tetrahedron Lett. 27, 1553–1556 (1986).
Kaszynki, P. & Michl, J. A practical photochemical synthesis of bicyclo[1.1.1]pentane-1,3-dicarboxylic acid. J. Org. Chem. 53, 4593–4594 (1988).
Gianatassio, R. et al. Strain-release amination. Science 351, 241–246 (2016).
Lopchuk, J. M. et al. Strain-release heteroatom functionalization: development, scope, and stereospecificity. J. Am. Chem. Soc. 139, 3209–3226 (2017).
Ma, X. & Pham, L. N. Selective topics in the syntheses of bicyclo[1.1.1]pentane (BCP) analogues. Asian J. Org. Chem. 9, 8–22 (2020).
Kanazawa, J. & Uchiyama, M. Recent advances in the synthetic chemistry of bicyclo[1.1.1]pentane. Synlett 30, 1–11 (2019).
Makarov, I. S., Brocklehurst, C. E., Karaghiosoff, K., Koch, G. & Knochel, P. Synthesis of bicyclo[1.1.1]pentane bioisosteres of internal alkynes and para-disubstituted benzenes from [1.1.1] propellane. Angew. Chem. Int. Ed. 56, 12774–12777 (2017).
Kanazawa, J., Maeda, K. & Uchiyama, M. Radical multicomponent carboamination of [1.1.1]propellane. J. Am. Chem. Soc. 139, 17791–17794 (2017).
Kondo, M. et al. Silaboration of [1.1.1]propellane: a storable feedstock for bicyclo[1.1.1]pentane derivatives. Angew. Chem. Int. Ed. 59, 1970–1974 (2020).
Caputo, D. F. J. et al. Synthesis and applications of highly functionalized 1-halo-3-substituted bicyclo[1.1.1]pentanes. Chem. Sci. 9, 5295–5300 (2018).
Nugent, J. et al. A general route to bicyclo[1.1.1]pentanes through photoredox catalysis. ACS Catal. 9, 9568–9574 (2019).
Zhang, X. et al. Copper-mediated synthesis of drug-like bicyclopentanes. Nature 580, 220–226 (2020).
Trongsiriwat, N. et al. Reactions of 2‐aryl‐1,3‐dithianes and [1.1.1]propellane. Angew. Chem. Int. Ed. 58, 13416–13420 (2019).
Hughes, J. M. E., Scarlata, D. A., Chen, A. C.-Y., Burch, J. & Gleason, J. L. Aminoalkylation of [1.1.1]propellane enables direct access to high-value 3-alkylbicyclo[1.1.1]pentan-1-amines. Org. Lett. 21, 6800–6804 (2019).
Shelp, R. A. & Walsh, P. J. Synthesis of BCP benzylamines from 2‐azaallyl anions and [1.1.1]propellane. Angew. Chem. Int. Ed. 57, 15857–15861 (2018).
Yu, S., Jing, C., Noble, A. & Aggarwal, V. K. 1,3-Difunctionalizations of [1.1.1]propellane via 1,2-metalate rearrangements of boronate complexes. Angew. Chem. Int. Ed. 59, 3917–3921 (2020).
Kim, J. H., Ruffoni, A., Al-Faiyz, Y. S. S., Sheikh, N. & Leonori, D. Divergent strain‐release amino‐functionalization of [1.1.1]propellane with electrophilic nitrogen‐radicals. Angew. Chem. Int. Ed. 59, 8225–8231 (2020).
Shin, S., Lee, S., Choi, W., Kim, N. & Hong, S. Visible-light-induced 1,3-aminopyridylation of [1.1.1]propellane with N-aminopyridinium salts. Angew. Chem. Int. Ed. 60, 7873–7879 (2021).
Toriyama, F. et al. Redox-active esters in Fe-catalyzed C–C coupling. J. Am. Chem. Soc. 138, 11132–11135 (2016).
VanHeyst, M. D. et al. Continuous flow-enabled synthesis of bench-stable bicyclo[1.1.1]pentane trifluoroborate salts and their utilization in metallaphotoredox cross-couplings. Org. Lett. 22, 1648–1654 (2020).
Garlets, Z. J. et al. Enantioselective C–H functionalization of bicyclo[1.1.1]pentanes. Nat. Catal. 3, 351–357 (2020).
Zarate, C. et al. Development of scalable routes to 1-bicyclo[1.1.1]pentylpyrazoles. Org. Process Res. Dev. 25, 642–647 (2021).
Shelp, R. A. et al. Strain-release 2-azaallyl anion addition/borylation of [1.1.1]propellane: synthesis and functionalization of benzylamine bicyclo[1.1.1]pentyl boronates. Chem. Sci. 12, 7066–7072 (2021).
Ma, X., Han, Y. & Bennett, D. J. Selective synthesis of 1-dialkylamino-2-alkylbicyclo-[1.1.1]pentanes. Org. Lett. 22, 9133–9138 (2020).
Zhao, J.-X. et al. 1,2-Difunctionalized bicyclo[1.1.1]pentanes: long-sought-after mimetics for ortho/meta-substituted arenes. Proc. Natl. Acad. Sci. USA 118, e2108881118 (2021).
Bychek, R. M. et al. Difluoro-substituted bicyclo[1.1.1]pentanes for medicinal chemistry: design, synthesis, and characterization. J. Org. Chem. 84, 15106–15117 (2019).
Ma, X., Sloman, D. L., Han, Y. & Bennett, D. J. A selective synthesis of 2,2-difluorobicyclo[1.1.1]pentane analogues: “BCP-F2”. Org. Lett. 21, 7199–7203 (2019).
Kaleta, J. et al. Bridge-chlorinated bicyclo[1.1.1]pentane-1,3-dicarboxylic acids. J. Org. Chem. 84, 2448–2461 (2019).
Wiberg, K. B., Connor, D. S. & Lampman, G. M. The reaction of 3-bromocyclobutane-1-methyl bromide with sodium: bicyclo[1.1.1]pentane. Tetrahedron Lett. 5, 531–534 (1964).
Padwa, A. & Alexander, E. Photochemical formation of a substituted bicyclo[1.1.1]pentane. J. Am. Chem. Soc. 89, 6376–6377 (1967).
Srinivasan, R. & Carlough, K. H. Mercury(3P1) photosensitized internal cycloaddition reactions in 1,4-, 1,5-, and 1,6-dienes. J. Am. Chem. Soc. 89, 4932–4936 (1967).
Applequist, D. E., Renken, T. L. & Wheeler, J. W. Polar substituent effects in 1,3-disubstituted bicyclo[1.1.1]pentanes. J. Org. Chem. 47, 4985–4995 (1982).
Meinwald, J., Szkryalo, W. & Dimmel, D. R. Bicyclo[1.1.1]pentane from mercury sensitized and unsensitized gas phase photolyses of bicyclo[2.1.1]hexan-2-one. Tetrahedron Lett. 8, 731–733 (1967).
Wiberg, K. B. in The Chemistry of Cyclobutanes Ch. 1, 1–15 (Wiley, 2005).
Wiberg, K. B. & Connor, D. S. Bicyclo[1.1.1]pentane. J. Am. Chem. Soc. 88, 4437–4441 (1966).
Yang, Y. et al. Practical and modular construction of C(sp3)-rich alkyl boron compounds. J. Am. Soc. Chem. 143, 471–480 (2021).
Barluenga, J., Tomás-Gamasa, M., Aznar, F. & Valdés, C. Metal-free carbon–carbon bond-forming reductive coupling between boronic acids and tosylhydrazones. Nat. Chem. 1, 494–499 (2009).
Préz-Aguilar, M. C. & Valdés, C. Olefination of carbonyl compounds through reductive coupling of alkenylboronic acids and tosylhydrazones. Angew. Chem. Int. Ed. 51, 5953–5957 (2012).
Plaza, M. & Valdés, C. Stereoselective domino carbocyclizations of γ- and δ-cyano-N-tosylhydrazones with alkenylboronic acids with formation of two different C(sp3)–C(sp2) bonds on a quaternary stereocenter. J. Am. Chem. Soc. 138, 12061–12064 (2016).
Plaza, M., Paraja, M., Florentino, L. & Valdés, C. Domino synthesis of benzo-fused β,γ-unsaturated ketones from alkenylboronic acids and N-tosylhydrazone-tethered benzonitriles. Org. Lett. 21, 632–635 (2019).
Wu, G., Deng, Y., Wu, C., Zhang, Y. & Wang, J. Synthesis of α-aryl esters and nitriles via deaminative coupling of α-aminoesters and α-aminoacetonitriles with arylboronic acids. Angew. Chem. Int. Ed. 53, 10510–10514 (2014).
Merchant, R. R. & Lopez, J. A. A general C(sp3)-C(sp3) cross-coupling of benzyl sulfonylhydrazones with alkyl boronic acids. Org. Lett. 22, 2271–2275 (2020).
Plaza, M., Parisotto, S. & Valdés, C. Heterocyclization and spirocyclization processes based on domino reactions of N‐tosylhydrazones and boronic acids involving intramolecular allylborylations of nitriles. Chem. Eur. J. 24, 14836–14843 (2018).
Arunprasath, D., Bala, B. D. & Sekar, G. Luxury of N-tosylhydrazones in transition-metal-free transformations. Adv. Synth. Catal. 361, 1172–1207 (2019).
Li, H., Wang, L., Zhang, Y. & Wang, J. Transition-metal-free synthesis of pinacol alkylboronates from tosylhydrazones. Angew. Chem. Int. Ed. 51, 2943–2946 (2012).
Tani, K. & Stoltz, B. M. Synthesis and structural analysis of 2-quinuclidonium tetrafluoroborate. Nature 441, 731–734 (2006).
Davenport, R. et al. Visible-light-driven strain-increase ring contraction allows the synthesis of cyclobutyl boronic esters. Angew. Chem. Int. Ed. 59, 6525–6528 (2020).
Hirsch, J. A. Table of conformational energies. Top. Stereochem. 1, 199–222 (1967).
Eliel, E. L., Wilen, S. H. & Doyle, M. P. in Basic Organic Stereochemistry 443 (Wiley, 2001).
Yang, C.-T. et al. Alkylboronic esters from copper‐catalyzed borylation of primary and secondary alkyl halides and pseudohalides. Angew. Chem. Int. Ed. 51, 528–532 (2012).
Stymiest, J. L., Dutheuil, G., Mahmood, A. & Aggarwal, V. K. Lithiated carbamates: chiral carbenoids for iterative homologation of boranes and boronic esters. Angew. Chem. Int. Ed. 46, 7491–7494 (2007).
Li, H. et al. Formal carbon insertion of N-tosylhydrazone into B–B and B–Si bonds: gem-diborylation and gem-silylborylation of sp3 carbon. Org. Lett. 16, 448–451 (2014).
Bonet, A., Pubill-Ulldemolins, C., Bo, C., Gulyás, H. & Fernández, E. Transition‐metal‐free diboration reaction by activation of diboron compounds with simple Lewis bases. Angew. Chem. Int. Ed. 50, 7158–7161 (2011).
Kisan, S., Krishnakumar, V. & Gunanathan, C. Ruthenium-catalyzed anti-Markovnikov selective hydroboration of olefins. ACS Catal. 7, 5950–5954 (2017).
Yamamoto, Y., Fujikawa, R., Umemoto, T. & Miyaura, N. Iridium-catalyzed hydro-boration of alkenes with pinacolborane. Tetrahedron 60, 10695–10700 (2004).
Fasano, V. et al. How big is the pinacol boronic ester as a substituent? Angew. Chem. Int. Ed. 59, 22403–22407 (2020).
Odachowski, M. et al. Development of enantiospecific coupling of secondary and tertiary boronic esters with aromatic compounds. J. Am. Chem. Soc. 138, 9521–9532 (2016).
Zweifel, G., Arzoumanian, H. & Whitney, C. C. A convenient stereoselective synthesis of substituted alkenes via hydroboration-iodination of alkynes. J. Am. Chem. Soc. 89, 3652–3653 (1967).
Armstrong, R. J. & Aggarwal, V. K. 50 years of Zweifel olefination: a transition-metal-free coupling. Synthesis 49, 3323–3336 (2017).
Sadhu, K. M. & Matteson, D. S. (Chloromethyl)lithium: efficient generation and capture by boronic esters and a simple preparation of diisopropyl (chloromethyl)boronate. Organometallics 4, 1687–1689 (1985).
Pozzi, D., Scanlan, E. M. & Renaud, P. A mild radical procedure for the reduction of B-alkylcatecholboranes to alkanes. J. Am. Chem. Soc. 127, 14204–14205 (2005).
André-Joyaux, E., Kuzovlev, A., Tappin, N. D. C. & Renaud, P. A general approach to deboronative radical chain reactions with pinacol alkylboronic esters. Angew. Chem. Int. Ed. 59, 13859–13864 (2020).
Littke, A. F., Dai, C. & Fu, G. C. Versatile catalysts for the Suzuki cross-coupling of arylboronic acids with aryl and vinyl halides and triflates under mild conditions. J. Am. Chem. Soc. 122, 4020–4028 (2000).
Dreher, S. D., Dormer, P. G., Sandrock, D. L. & Molander, G. A. Efficient cross-coupling of secondary alkyltrifluoroborates with aryl chlorides—reaction discovery using parallel microscale experimentation. J. Am. Chem. Soc. 130, 9257–9259 (2008).
Li, L., Zhao, S., Joshi-Pangu, A., Diane, M. & Biscoe, M. R. Stereospecific Pd-catalyzed cross-coupling reactions of secondary alkylboron nucleophiles and aryl chlorides. J. Am. Chem. Soc. 136, 14027–14030 (2014).
Brown, H. C., Midland, M. M. & Levy, A. B. Facile reaction of alkyl- and aryldichloroboranes with organic azides. General stereospecific synthesis of secondary amines. J. Am. Chem. Soc. 95, 2394–2396 (1973).
Bagutski, V., Elford, T. G. & Aggarwal, V. K. Synthesis of highly enantioenriched C‐tertiary amines from boronic esters: application to the synthesis of igmesine. Angew. Chem. Int. Ed. 50, 1080–1083 (2011).
Kuduk, S. D. & Skudlarek, J. W. 2-Pyridyloxy-3-substituted-4-nitrile orexin receptor antagonists. WO patent WO2014066196A1 (2014).
Acknowledgements
Financial support for this work was provided by the Welch Foundation (I-2010-20190330), the National Institutes of Health (R01GM141088) and UT Southwestern Eugene McDermott Endowed Scholarship. We thank F. Lin (UTSW) for assistance with NMR spectroscopy, H. Baniasadi (UTSW) for HRMS and V. Lynch (UT-Austin) for X-ray crystallographic analysis. We thank the Chen, Tambar, Ready, De Brabander, Smith and Falck groups (UTSW) for generous access to equipment, and helpful discussions. We are grateful to K. Campos, L.-C. Campeau, P. Fier, C. Zarate Saez and K. McClymont (Merck & Co., Inc., Kenilworth, NJ, USA) for feedback on this manuscript.
Author information
Authors and Affiliations
Contributions
Y.Y., J.T. and T.Q. performed the experiments; J.M.E.H., B.K.P., R.R.M. and T.Q. designed and supervised the project; J.M.E.H. and B.K.P. performed the DSC experiments; Y.Y., J.T., J.M.E.H., R.R.M. and T.Q. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
A provisional patent application naming T.Q., Y.Y. and J.T. as inventors has been filed by the Board of Regents of the University of Texas System, which covers the synthetic method and structural motifs described in this manuscript. The remaining authors declare no competing interests.
Additional information
Peer review information Nature Chemistry thanks Varinder Aggarwal, Cara Brocklehurst and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information
General experimental, general procedures, additional optimization of cyclizations, Suzuki cross-couplings and aminations, limitations, troubleshooting, experimental procedures and characterization data of starting materials, substrate precursors and substrates, DSC experiments, X-ray crystallographic data and NMR spectra.
Supplementary Data 1
Crystallographic data for compound 16; CCDC reference 2062923.
Supplementary Data 2
Crystallographic data for compound 20; CCDC reference 2062924.
Supplementary Data 3
Crystallographic data for compound 26; CCDC reference 2062925.
Supplementary Data 4
Crystallographic data for compound 40-ester; CCDC reference 2081087.
Rights and permissions
About this article
Cite this article
Yang, Y., Tsien, J., Hughes, J.M.E. et al. An intramolecular coupling approach to alkyl bioisosteres for the synthesis of multisubstituted bicycloalkyl boronates. Nat. Chem. 13, 950–955 (2021). https://doi.org/10.1038/s41557-021-00786-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-021-00786-z
This article is cited by
-
Ni-catalysed assembly of axially chiral alkenes from alkynyl tetracoordinate borons via 1,3-metallate shift
Nature Chemistry (2024)
-
C−F bond activation enables synthesis of aryl difluoromethyl bicyclopentanes as benzophenone-type bioisosteres
Nature Communications (2024)
-
Programmable late-stage functionalization of bridge-substituted bicyclo[1.1.1]pentane bis-boronates
Nature Chemistry (2024)
-
2-Oxabicyclo[2.1.1]hexanes as saturated bioisosteres of the ortho-substituted phenyl ring
Nature Chemistry (2023)
-
Catalytic 4-exo-dig carbocyclization for the construction of furan-fused cyclobutanones and synthetic applications
Nature Communications (2023)