Abstract
Ever since Hirata’s report of yuzurimine in 1966, nearly fifty yuzurimine-type alkaloids have been isolated, which formed the largest subfamily of the Daphniphyllum alkaloids. Despite extensive synthetic studies towards this synthetically challenging and biologically intriguing family, no total synthesis of any yuzurimine-type alkaloids has been achieved to date. Here, the first enantioselective total synthesis of (+)-caldaphnidine J, a highly complex yuzurimine-type Daphniphyllum alkaloid, is described. Key transformations of this approach include a highly regioselective Pd-catalyzed hydroformylation, a samarium(II)-mediated pinacol coupling, and a one-pot Swern oxidation/ketene dithioacetal Prins reaction. Our approach paves the way for the synthesis of other yuzurimine-type alkaloids and related natural products.
Similar content being viewed by others
Introduction
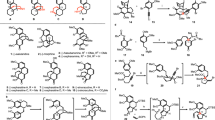
The plant family of genus Daphniphyllum has produced a wide range of complex caged natural products—the Daphniphyllum alkaloids1,2,3,4. Owing to their challenging chemical structures and interesting biological profiles (such as anticarcinogenic, neurotrophic, and anti-HIV activity)5,6, these alkaloids have drawn much attention from the synthetic community1,3,4. Depending on the taxonomy, the diversified structures of the Daphniphyllum alkaloids can be categorized into 13–35 subfamilies1,4,7. To date, about 20 elegant total syntheses of the Daphniphyllum alkaloids, from seven subfamilies, have been reported by the research groups of Heathcock8,9,10,11,12,13,14,15, Carreira16, Li17,18,19,20, Smith21,22, Hanessian23, Fukuyama/Yokoshima24, Zhai25, Dixon26, Qiu27, Gao28, and Sarpong29,30 groups, respectively. Moreover, our group has recently accomplished the asymmetric total syntheses of himalensine A31, dapholdhamine B32, and caldaphnidine O33. However, despite extensive synthetic studies34,35,36,37,38,39,40,41,42,43,44,45,46,47,48, total synthesis of yuzurimine-type alkaloids—the largest subfamily of the Daphniphyllum alkaloids—has not been achieved so far.
Since the milestone achievement by Hirata in 196649, nearly 50 yuzurimine-type alkaloids were isolated, which account for almost one-sixth of all known Daphniphyllum alkaloids to date. It is recognized that the members of this subfamily share a unique, highly complex and caged hexacyclic skeleton (Fig. 1). Caldaphnidine J, our target molecule, was isolated by the Yue group in 200850. It possesses a hexacyclic scaffold with six contiguous stereogenic centers, two quaternary centers, and an α,β,γ,δ-unsaturated carboxylic ester thus signifying a formidable synthetic challenge. Following our long-lasting interest in the synthesis of the Daphniphyllum alkaloids31,32,33,51,52, we now wish to report an asymmetric total synthesis of yuzurimine-type alkaloid, (+)-caldaphnidine J. Our approach is featured with a Pd-catalyzed regioselective hydroformylation that furnishes the critical aldehyde motif, and the construction of the 5/5 bicyclic system using a SmI2-promoted pinacol coupling and a one-pot Swern oxidation/ketene dithioacetal Prins reaction. Other notable attempts toward the 7/5 bicycle in our target molecule are also discussed.
Results
Retrosynthetic analysis
As shown in Fig. 2, our retrosynthetic analysis indicates that (+)-caldaphnidine J could be converted from the intermediate 1 via Ni(0)-mediated reductive coupling reaction53. It was further envisioned that a ketene dithioacetal Prins reaction54,55,56,57 would form the cyclopentene moiety in 1, while compound 2 might be produced from the enyne acetal 3 via a carbocyclization cascade58. Lastly, compound 3 could be synthesized via a facile ring cleavage from the readily available chiral building block, tricyclic ketone 433.
Initial synthetic studies toward the 7/5 ring system
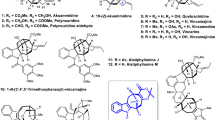
The first stage of our investigation focused on the critical 7/5 ring scaffold assembly (Fig. 3). The treatment of the known tricycle 433 with MeMgBr in the presence of CeCl3 was followed by an oxidative cleavage of the intermediate diol producing dicarbonyl 5. Subsequently, 5 was subjected to Wittig conditions to yield the enol methyl ether, which was then transformed into acetal 6. Following Stang’s protocol59, ketone motif in 6 was converted into an enol triflate, which was then treated with pyridine affording alkyne 7. Subsequent Sonogashira coupling yielded enyne acetal 3. Inspired by Saá’s impressive Nazarov carbocyclization cascade58, enyne acetal 3 was treated with Brønsted acids such as TFA or HBF4•OEt2. However, none of the desired hydroazulenone 8 was detected. Alternatively, attempting to use a formic acid-promoted cyclization60,61 between the thioalkyne and the acetal motifs in compound 9 returned only messy mixtures. It was postulated that the desired cyclization was prevented by the steric bulk at C-8 (caldaphnidine J numbering).
On the other hand, aldol condensation of 5 yielded solely cyclopentene 11, but not cycloheptenone 12 possessing the desired seven-membered ring (Fig. 3). Instead, selective reduction of the aldehyde motif in 5 followed by an Appel reaction afforded the corresponding alkyl iodide, which then underwent an intramolecular alkylation upon treatment with LDA yielding the desired tricycle 13 in 69% overall yield. Inspired by Smith’s impressive work21,22, it was envisioned that a carbonylative Stille coupling reaction followed by a Nazarov cyclization would elaborate the desired bicycle 8. While ketone 13 is quite similar to Smith’s substrates21,22, in our hands all of the deprotonation attempts failed resulting in no reaction at negative and decomposition events at elevated temperatures. It is quite possible that the corresponding enol triflate 14 is unstable by introducing increased ring strain to the 6/6/7 tricyclic system.
Total synthesis of (+)-caldaphnidine J
The fruitless synthetic attempts toward the 7/5 bicyclic ring scaffold forced us to use the similar strategy to the total synthesis of caldaphnidine O33. As depicted in Fig. 4, treatment of ketone 4 with AllylMgBr in the presence of CeCl3 furnished a diol intermediate, which was subjected to a Pb(IV)-mediated oxidative cleavage. A follow-up NaBH4 reduction yielded a somewhat unstable β,γ-unsaturated ketone 16, which was immediately subjected to iodination producing alkyl iodide 17. Subsequently, treating 17 with LDA triggered an intramolecular alkylation to afford α-vinyl functionalized ketones 18a (minor, 25%) and 18b (major, 50%). The stereoconfigurations of 18a and 18b were assigned at later stage, according to a single-crystal X-ray diffraction (XRD) data of compound 22. Initially, we envisioned to subject both isomers of 18 to a Pd-catalyzed carbonylative cyclization62,63, however preparation of the corresponding enol triflate failed. Alternatively, the terminal alkene motif in 18b was regioselectively hydroformylated following Shi’s protocol furnishing aldehyde 19 in 75% yield64. Any branched side products were not detected during this transformation. Although aldehyde 19 could also be synthesized via rather routine methods33, the intrinsic structural complexity of substrate 18b enabled the exciting opportunity for expanding the substrate scope for Shi’s regioselective hydroformylation. Subsequently, an intramolecular pinacol coupling reaction mediated by SmI2 furnished diol 20, which possesses the essential 7/5 bicyclic ring scaffold. Selective acylation of the secondary hydroxyl, followed by DDQ-mediated debenzylation afforded primary alcohol 21, which was then converted into aldehyde 22 using Dess-Martin oxidation. The identity of 22 was unambiguously confirmed via XRD analysis. A Horner–Wadsworth–Emmons homologation using phosphonate 2332,33,65, followed by one-pot DIBAL-H reduction afforded ketene dithioacetal 25 in 94% yield. Notably, the intermediate acetate 24 gave a single crystal suitable for XRD analysis.
Key transformations were highlighted in colored text. Ac acetyl, AIBN azodiisobutyronitrile, DBU 1,8-diazabicyclo[5.4.0]undec-7-ene, DCE dichloroethane, DDQ 2,3-dichloro-5,6-dicyano-1,4-benzoquinone, DIBAL-H diisobutylaluminium hydride, DMAP 4-dimethylaminopyridine, DME 1,2-dimethoxyethane, dppp 1,3-bis(diphenylphosphino) propane, Naph. naphthalene, TFAA trifluoroacetic anhydride.
At this stage, aldol-type16,21,22 or Prins-type reaction was required to form the critical C14–C15 bond in 26. However, ketene dithioacetal-involved aldol- or Prins-type reactions are poorly explored. There are only a handful of examples reported in an intermolecular manner54,55,56,57. Inspired by these investigations, it was envisaged that oxidation of the hydroxyl at C-15 would produce the corresponding ketone motif, and the following ketene dithioacetal Prins-type reaction should form the crucial C–C bond thus constructing the desired pentacycle. To our delight, subjecting diol 25 to a TFAA/DMSO-mediated Swern oxidation triggered the desired cyclization of the dithioacetal ketene onto a newly formed ketone moiety. The ultimate product 26 was then formed by 1,2-addition of dimethyl sulfide onto the sulfonium intermediate, followed by demethylation66. While all reported examples of intermolecular ketene dithioacetal aldol/Prins-type reactions were performed under strong Lewis acid conditions such as BF3•Et2O, TiCl4, and TMSOTf54,55,56,57, the addition of a strong Lewis acid were not necessary for our ketone intermediate derived from 25. Moreover, using other oxidants such as (COCl)2/DMSO, Py•SO3/DMSO, NCS/DMSO, or Ac2O/DMSO resulted in decomposition of 25 giving poorly identifiable side products. The 2-(methylthio)-1,3-dithiane moiety in 26 was then smoothly transformed to the methyl ester 27 by the action of methanolic iodine. Selective elimination of the hydroxyl at C-15 of 27 was required to avoid any possible side reaction between the α-aminyl radical and the C9–C10 alkene moiety in a later stage of our synthesis33. Gratifyingly, treating cis-diol 27 with SOCl2 yielded the dialkyl sulfite 28, which upon treatment with DBU suffered E2cB elimination providing the hope-for allylic alcohol 29. Removal of the tosyl appendage in 29 was followed by a one-pot alkylation to yield vinyl bromide 30. This vinyl bromide substrate enabled the investigation of the Ni(0)-catalyzed C–C-coupling and the Tin-mediated radical cyclization approaches. A Ni(0)-mediated reductive coupling of 3053, unfortunately, failed to produce 31 but only messy results. There was not any identifiable product that could be isolated. LC-MS indicates only a trace amount of the desired hexacycle 31. Alternatively, subjecting 30 to an AIBN/Bu3SnH -mediated radical cyclization assembled the key tetrahydropyrrole ring in 31. The acidic workup promoted the elimination of the C-9 hydroxyl group to yield the conjugated diene. Lastly, a highly regio- and diastereoselective hydrogenation (H2/Ar = 1:1, Crabtree’s catalyst) of 31 successfully produced (+)-caldaphnidine J in 81% yield (dr = 8:1). The synthetic (+)-caldaphnidine J gave spectral characteristics (1H- and 13C-NMR spectroscopy and HRMS data) consistent with those of the naturally occurring (+)-caldaphnidine J, while the optical rotation is also in perfect agreement with that of the natural product (synthetic: [α]D20 = + 60.0 (c = 0.1 in MeOH); natural: [α]D20 = + 57.0 (c = 0.2 in MeOH)50.
Discussion
Owing to their extremely challenging structures, the synthesis of yuzurimine-type Daphniphyllum alkaloids remain unexplored for more than half a century. These intriguing alkaloids provide ideal platforms for developing and probing various synthetic strategies and methods. In this paper, we have accomplished an asymmetric synthesis of highly challenging yuzurimine-type Daphniphyllum alkaloid (+)-caldaphnidine J in 17 steps from readily available chiral synthon (+)−4. This work achieves the synthesis of a member of the largest yet unexplored subfamily of Daphniphyllum alkaloids. The highlights of our synthesis include: (1) a highly regioselective Pd-catalyzed hydroformylation reaction; (2) a Sm(II)-mediated pinacol coupling that produced a highly challenging 7/5 bicyclic system while all other attempts failed; (3) a one-pot Swern oxidation/ketene dithioacetal Prins reaction; (4) a regioselective elimination through a cyclic sulfite intermediate, and (5) a radical cyclization reaction that rapidly constructed the tetrahydropyrrole motif. The synthetic strategies and methods should inspire further advances in the synthesis of diverse Daphniphyllum alkaloids and related natural products.
Methods
General
Unless indicated, all commercially available reagents and anhydrous solvents were purchased at the highest commercial quality and were used as received without further purification. All non-aqueous reactions were carried out under argon atmosphere using dry glassware that had been flame-dried under a stream of argon unless otherwise noted. Tetrahydrofuran (THF) was distilled from sodium benzophenone under argon atmosphere. Dichloromethane (DCM) was distilled from calcium hydride. Reactions were monitored by thin-layer chromatography (TLC; GF254) using plates supplied by Yantai Chemicals (China) and visualized under UV or by staining with an ethanolic solution of phosphomolybdic acid, cerium sulfate or iodine. Flash column chromatography was performed using silica gel (particle size, 0.040–0.063 mm).
NMR spectra were recorded on a Bruker AV400 MHz or a Bruker AscendTM 500 MHz instrument and calibrated using residual undeuterated chloroform, methanol, and dichloromethane in CDCl3 (δ H = 7.26 ppm, δ C = 77.0 ppm), MeOD-d4 (δ H = 3.31 ppm, δ C = 49.0 ppm), or CD2Cl2 (δ H = 5.32 ppm, δ C = 53.84 ppm) as an internal reference. The following abbreviations were used to describe signal multiplicities: s, singlet; d, doublet; t, triplet; dt, double triplet; ddd, doublet of double doublet; ddt, doublet of double triplet; m, multiplet. High-resolution mass spectra (HRMS) were recorded on a Thermo Scientific Q Exactive Hybrid Quadrupole Orbitrap mass spectrometer.
Experimental data
For NMR spectra of synthetic intermediates, see Supplementary Figs. 3–57. For the experimental procedures and spectroscopic and physical data of compounds and the crystallographic data of compound 22 and 24, see Supplementary Methods.
Supplementary Information accompanies this manuscript.
Data availability
The X-ray crystallographic coordinates for structures 22 and 24 reported in this study have been deposited at the Cambridge Crystallographic Data Centre (CCDC) with the accession codes CCDC 1986889 and CCDC 1986960 (www.ccdc.cam.ac.uk/data_request/cif), respectively. We declare that all other relevant data supporting the findings of this study are available within the article and its Supplementary Information files.
References
Kobayashi, J. & Kubota, T. The Daphniphyllum alkaloids. Nat. Prod. Rep. 26, 936–962 (2009).
Yang, S.-P. & Yue, J.-M. Discovery of structurally diverse and bioactive compounds from plant resources in China. Acta Pharmacol. Sin. 33, 1147–1158 (2012).
Kang, B., Jakubec, P. & Dixon, D. J. Strategies towards the synthesis of calyciphylline A-type Daphniphyllum alkaloids. Nat. Prod. Rep. 31, 550–562 (2014).
Chattopadhyay, A. K. & Hanessian, S. Recent progress in the chemistry of Daphniphyllum alkaloids. Chem. Rev. 117, 4104−4146 (2017).
Wu, H. et al. Daphniphyllum alkaloids: recent findings on chemistry and pharmacology. Planta Med. 79, 1589–1598 (2013).
Xu, J.-B. et al. Logeracemin A, an anti-HIV Daphniphyllum alkaloid dimer with a new carbon skeleton from Daphniphyllum longeracemosum. J. Am. Chem. Soc. 136, 7631–7633 (2014).
Li, Z.-Y. & Guo, Y.-W. Progress in the study of Daphniphyllum alkaloids. Youji Huaxue 27, 565 (2007).
Piettre, S. & Heathcock, C. H. Biomimetic total synthesis of proto-daphniphylline. Science 248, 1532–1534 (1990).
Heathcock, C. H., Davidsen, S. K., Mills, S. & Sanner, M. A. Total synthesis of (±)-methyl homodaphniphyllate. J. Am. Chem. Soc. 108, 5650–5651 (1986).
Ruggeri, R. B. & Heathcock, C. H. Daphniphyllum alkaloids. Part 7. Biomimetic total synthesis of (±)-methyl homodaphniphyllate. J. Org. Chem. 55, 3714–3715 (1990).
Heathcock, C. H., Kath, J. C. & Ruggeri, R. B. Daphniphyllum alkaloids. 16. Total synthesis of (+)-codaphniphylline. J. Org. Chem. 60, 1120–1130 (1995).
Ruggeri, R. B., Hansen, M. M. & Heathcock, C. H. Total synthesis of (±)-methyl homosecodaphniphyllate. A remarkable new tetracyclization reaction. J. Am. Chem. Soc. 110, 8734–8736 (1988).
Stafford, J. A. & Heathcock, C. H. Daphniphyllum alkaloids. Part 8. Asymmetric total synthesis of (−)-secodaphniphylline. J. Org. Chem. 55, 5433–5434 (1990).
Heathcock, C. H., Stafford, J. A. & Clark, D. L. Daphniphyllum alkaloids. 14. Total synthesis of (±)-bukittinggine. J. Org. Chem. 57, 2575–2585 (1992).
Ruggeri, R. B., McClure, K. F. & Heathcock, C. H. Daphniphyllum alkaloids. Part 5. Total synthesis of (±)-daphnilactone A: a novel fragmentation reaction. J. Am. Chem. Soc. 111, 1530–1531 (1989).
Weiss, M. E. & Carreira, E. M. Total synthesis of (+)-daphmanidin E. Angew. Chem. Int. Ed. 50, 11501–11505 (2011).
Lu, Z. Y., Li, Y., Deng, J. & Li, A. Total synthesis of the Daphniphyllum alkaloid daphenylline. Nat. Chem. 5, 679–684 (2013).
Li, J., Zhang, W. H., Zhang, F., Chen, Y. & Li, A. Total synthesis of longeracinphyllin A. J. Am. Chem. Soc. 139, 14893–14896 (2017).
Chen, Y., Zhang, W. H., Ren, L., Li, J. & Li, A. Total syntheses of daphenylline, daphnipaxianine A, and himalenine D. Angew. Chem. Int. Ed. 57, 952−956. (2018)
Zhang, W. H. et al. Total synthesis of hybridaphniphylline B. J. Am. Chem. Soc. 140, 4227–4231 (2018).
Shvartsbart, A. & Smith, A. B. III Total Synth. (−)-calyciphylline N. J. Am. Chem. Soc. 136, 870–873 (2014).
Shvartsbart, A. & Smith, A. B. III. Daphniphyllum alkaloids: Total Synth. (−)-calyciphylline N. J. Am. Chem. Soc. 137, 3510–3519 (2015).
For the synthesis of a putative member of calyciphylline B-type alkaloids, see: Chattopadhyay, A. K., Ly, V. L., Jakkepally, S., Berger, G. & Hanessian, S. Total synthesis of isodaphlongamine H: a possible biogenetic conundrum. Angew. Chem. Int. Ed. 55, 2577−2581 (2016).
Yamada, R., Adachi, Y., Yokoshima, S. & Fukuyama, T. Total synthesis of (−)-daphenylline. Angew. Chem. Int. Ed. 55, 6067–6070 (2016).
Chen, X. et al. Divergent total syntheses of (−)-daphnilongeranin B and (−)-daphenylline. Angew. Chem. Int. Ed. 57, 947–951 (2018).
Shi, H. et al. Total synthesis of (−)-himalensine A. J. Am. Chem. Soc. 139, 17755–17758 (2017).
Xu, B., Wang, B., Xun, W. & Qiu, F. G. Total synthesis of (−)-daphenylline. Angew. Chem. Int. Ed. 58, 5754–5757 (2019).
Zhong, J., Chen, K., Qiu, Y., He, H. & Gao, S. A unified strategy to construct the tetracyclic ring of Calyciphylline A alkaloids: total synthesis of himalensine A. Org. Lett. 21, 3741–3745 (2019).
Hugelshofer, C. L., Palani, V. & Sarpong, R. Calyciphylline B type alkaloids: total syntheses of (−)-daphlongamine H and (−)-isodaphlongamine H. J. Am. Chem. Soc. 141, 8431–8435 (2019).
Hugelshofer, C. L., Palani, V. & Sarpong, R. Calyciphylline B-type alkaloids: evolution of a synthetic strategy to (–)-daphlongamine H. J. Org. Chem. 84, 14069–14091 (2019).
Chen, Y. et al. A concise total synthesis of (−)-himalensine A. Angew. Chem. Int. Ed. 58, 7390–7394 (2019).
Guo, L.-D. et al. Total synthesis of dapholdhamine B and dapholdhamine B lactone. J. Am. Chem. Soc. 141, 11713–11720 (2019).
Guo, L.-D. et al. Enantioselective total synthesis of (−)-caldaphnidine O via a radical cyclization cascade. J. Am. Chem. Soc. 141, 13043–13048 (2019).
Kitabayashi, Y., Fukuyama, T. & Yokoshima, S. Synthesis of the [7-5-5] tricyclic core of Daphniphyllum alkaloids. Org. Biomol. Chem. 16, 3556–3559 (2018).
Hayakawa, R. et al. Toward the synthesis of yuzurimine-type alkaloids: stereoselective construction of the heterocyclic portions of deoxyyuzurimine and macrodaphnine. Org. Lett. 21, 6337–6341 (2019).
Solé, D., Urbaneja, X. & Bonjoch, J. Synthesis of the 4-azatricyclo[5.2.2.0(4,8)]undecan-10-one core of daphniphyllum alkaloid calyciphylline A using a Pd-catalyzed enolate alkenylation. Org. Lett. 7, 5461–5464 (2005).
Cordero-Vargas, A., Urbaneja, X. & Bonjoch, J. A stereocontrolled entry to 3-functionalized cis-3a-methyloctahydroindoles: building blocks for daphniphyllum alkaloid synthesis. Synlett, 2007, 2379–2382 (2007).
Denmark, S. E. & Baiazitov, R. Y. Tandem double-intramolecular [4+2]/[3+2] cycloadditions of nitroalkenes. studies toward a total synthesis of daphnilactone B: piperidine ring construction. J. Org. Chem. 71, 593–605 (2006).
Denmark, S. E., Nguyen, S. T. & Baiazitov, R. Y. Asymmetric synthesis of the ABCD ring system of daphnilactone B via a tandem, double intramolecular, [4+2]/[3+2]cycloaddition strategy. Heterocycles 76, 143–154 (2008).
Denmark, S. E., Baiazitov, R. Y. & Nguyen, S. T. Tandem double intramolecular [4+2]/[3+2] cycloadditions of nitroalkenes: construction of the pentacyclic core structure of daphnilactone B. Tetrahedron 65, 6535–6548 (2009).
Coldham, I., Burrell, A. J. M., Guerrand, H. D. S. & Oram, N. Cascade cyclization, dipolar cycloaddition to bridged tricyclic amines related to the Daphniphyllum alkaloids. Org. Lett. 13, 1267–1269 (2011).
Coldham, I., Watson, L., Adams, H. & Martin, N. G. Synthesis of the core ring system of the yuzurimine-type daphniphyllum alkaloids by cascade condensation, cyclization, cycloaddition chemistry. J. Org. Chem. 76, 2360–2366 (2011).
Bélanger, G., Boudreault, J. & Lévesque, F. Synthesis of the tetracyclic core of Daphnilactone B-type and yuzurimine-type alkaloids. Org. Lett. 13, 6204–6207 (2011).
Darses, B. et al. Expedient construction of the [7–5–5] all-carbon tricyclic core of the Daphniphyllum alkaloids Daphnilongeranin B and Daphniyunnine D. Org. Lett. 14, 1684–1687 (2012).
Yang, M. et al. Tandem semipinacol-type 1,2-carbon migration/aldol reaction toward the construction of [5–6–7] all-carbon tricyclic core of Calyciphylline A-type alkaloids. Org. Lett. 14, 5114–5117 (2012).
Yao, Y. & Liang, G. Rapid construction of the ABC ring system in the Daphniphyllum alkaloid Daphniyunnine C. Org. Lett. 14, 5499–5501 (2012).
Ibrahim, A. A., Golonka, A. N., Lopez, A. M. & Stockdill, J. L. Rapid access to the heterocyclic core of the Calyciphylline A and Daphnicyclidin A-type daphniphyllum alkaloids via tandem cyclization of a neutral aminyl radical. Org. Lett. 16, 1072–1075 (2014).
Kotha, S. & Ravikumar, O. Diversity-oriented approach to carbocycles and heterocycles through ring-rearrangement metathesis, fischer indole cyclization, and diels–alder reaction as key steps. Eur. J. Org. Chem. 2014, 5582–5590 (2014).
Sakurai, H., Sakabe, N. & Hirata, Y. X-ray structure determination of yuzurimine hydrobromide. Tetrahedron Lett. 7, 6309–6314 (1966).
Zhang, C.-R., Yang, S.-P. & Yue, J.-M. Alkaloids from the twigs of Daphniphyllum calycinum. J. Nat. Prod. 71, 1663–1668 (2008).
Chen, Y. et al. Synthesis of the core structure of Daphnimacropodines. Org. Lett. 21, 4309–4312 (2019).
Zhang, Y., Guo, L.-D. & Xu, J. Efficient synthesis of the AC ring system of Daphnilactone B. Youji Huaxue. 39, 1079–1084 (2019).
Solé, D., Cancho, Y., Llebaria, A., Moretó, J. M. & Delgado, A. Intramolecular nitrogen assistance in the nickel-promoted tandem cyclization-capture of amino-tethered vinyl bromides and alkenes. J. Am. Chem. Soc. 116, 12133–12134 (1994).
Chamberlin, A. R. & Chung, J. Enantioselective synthesis of seven pyrrolizidine diols from a single precursor. J. Org. Chem. 50, 4425–4431 (1985).
Tominaga, Y., Matsuoka, Y., Kamio, C. & Hosomi, A. Ketene dithioacetals in organic synthesis: synthesis of silyl ketene dithioacetal and some reactions with substituted benzaldehydes. Chem. Pharm. Bull. 37, 3168–3170 (1989).
Okauchi, T., Tanaka, T. & Minami, T. Lewis Acid-Promoted Deoxygenative Di[β,β-bis(ethylthio)]vinylation of Aldehydes with Trimethylsilylketene Bis(ethylthio)acetal. J. Org. Chem. 66, 3924–3929 (2001).
Saitoh, T., Jimbo, N. & Ichikawa, J. A novel synthesis of syn and anti β-hydroxy dithioacetals, masked cross-aldols between aldehydes. Chem. Lett. 33, 1032–1033 (2004).
Escalante, L., González-Rodríguez, C., Varela, J. & Saá, C. Tandem brønsted acid promoted and nazarov carbocyclizations of enyne acetals to hydroazulenones. Angew. Chem. Int. Ed. 51, 12316–12320 (2012).
Hargrove, R. & Stang, P. Vinyl triflates in synthesis. I. tert-Butylacetylene. J. Org. Chem. 39, 581–582 (1974).
Cuthbertson, J., Godfrey, A. & Taylor, R. The preparation of (−)-Grandisine B from (+)-Grandisine D; a biomimetic total synthesis or formation of an isolation artefact? Org. Lett. 13, 3976–3979 (2011).
Cuthbertson, J., Unsworth, W., Moody, C. & Taylor, R. The total synthesis of (+)-elaeokanidine A: natural product or isolation artefact? Tetrahedron Lett. 56, 3123–3126 (2015).
Gagnier, S. & Larock, R. Palladium-catalyzed carbonylative cyclization of unsaturated aryl iodides and dienyl triflates, iodides, and bromides to indanones and 2-cyclopentenones. J. Am. Chem. Soc. 125, 4804–4807 (2003).
Zeng, M., Murphy, S. & Herzon, S. Development of a modular synthetic route to (+)-pleuromutilin, (+)-12-epi-mutilins, and related structures. J. Am. Chem. Soc. 139, 16377–16388 (2017).
Ren, W. et al. An effective Pd-catalyzed regioselective hydroformylation of olefins with formic acid. J. Am. Chem. Soc. 138, 14864–14867 (2016).
Mikołajczyk, M. et al. Organosulphur compounds—XVIII1: A new and general synthesis of ketene s,s- and o,s-thioacetals based on the horner-wittig reaction. Tetrahedron 34, 3081–3088 (1978).
Trost, B. & Kunz, R. Methods in alkaloid synthesis. Imino ethers as donors in the Michael reaction. J. Am. Chem. Soc. 97, 7152–7157 (1975).
Acknowledgements
Financial support from Shenzhen Science and Technology Innovation Committee (JCYJ20170817110515599, KQJSCX20170728154233200, and KQTD20150717103157174), NSFC (21772082 and 21971104), Shenzhen Nobel Prize Scientists Laboratory Project (C17783101), Guangdong Innovative Program (No. 2019BT02Y335) and Guangdong Provincial Key Laboratory of Catalysis (No. 2020B121201002) are greatly appreciated. We also thank SUSTech CRF NMR facility, Dr. Xiaoyong Chang (SUSTech) for XRD analysis and Dr. Yang Yu (SUSTech) for HRMS analysis.
Author information
Authors and Affiliations
Contributions
J.X. conceived and designed the project and wrote the paper with assistance from L.-D.G., Y.Z., and J.H.; L.-D.G., Y.Z, J.H., C.N., H.F., and Y.C. performed the experiments. All authors discussed the results and commented on the manuscript. Y.Z. and J.H. contributed equally to this research.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Guo, LD., Zhang, Y., Hu, J. et al. Asymmetric total synthesis of yuzurimine-type Daphniphyllum alkaloid (+)-caldaphnidine J. Nat Commun 11, 3538 (2020). https://doi.org/10.1038/s41467-020-17350-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-020-17350-x
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.