Abstract
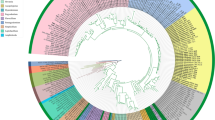
Botryosphaeriales was introduced in 2006 for a single family Botryosphaeriaceae. Since then the number of families has increased as a result of the transfer of one family (Planistromellaceae) into the order, re-instatement of another (Phyllostictaceae), while others resulted from raising genera to family status (Aplosporellaceae, Endomelanconiopsisaceae, Melanopsaceae, Pseudofusicoccumaceae, Saccharataceae and Septorioideaceae). All these decisions were based solely on phylogenetic analyses of several different loci. There has been no consensus on which loci are suitable markers at this taxonomic level and in some cases the datasets used to construct the phylogenies were incomplete. In this paper, the families of Botryosphaeriales were re-assessed in terms of morphology of the sexual morphs, phylogenetic relationships based on ITS and LSU sequence data, and evolutionary divergence times of lineages in relation to major events in the evolution of their hosts on a geological timescale. Six main lineages were resolved in the phylogenetic analyses and these correspond to six groups as defined on morphology of the sexual morphs. These lineages evolved during the Late epoch of the Cretaceous period and survived the catastrophic event that led to the mass extinction of non-avian dinosaurs and a great loss of plant diversity at the end of the Cretaceous period. They then diversified during the Paleocene and Eocene epochs of the Paleogene period. These six lineages are considered to represent families in Botryosphaeriales. Therefore, six families (Aplosporellaceae, Botryosphaeriaceae, Melanopsaceae, Phyllostictaceae, Planistromellaceae and Saccharataceae) are accepted in Botryosphaeriales, while three (Endomelanconiopsisaceae, Pseudofusicoccumaceae and Septorioideaceae) are reduced to synonymy under existing families.







Similar content being viewed by others
References
Alvarez LW, Alvarez W, Asaro F, Michel HV (1980) Extraterrestrial cause for the Cretaceous-Tertiary extinction. Science, New Series 208:1095–1108
Barr ME (1972) Preliminary studies on the Dothideales in temperate North America. Contrib Univ Michigan Herb 9:523–638
Barr ME (1976) Perspectives in the Ascomycotina. Mem N Y Bot Gard 28:1–8
Barr ME (1979) A classification of Loculoascomycetes. Mycologia 71:935–957
Barr ME (1987) Prodromus to class Loculoascomycetes. Published by the author, Amherst
Barr ME (1996) Planistromellaceae, a new family in the Dothideales. Mycotaxon 60:433–442
Berbee ML, Taylor JW (2010) Dating the molecular clock in fungi—how close are we? Fungal Biol Rev 24:1–16
Boonmee S, Zhang Y, Chomnunti P, Chukeatirote E, Tsui CKM, Bahkali AH, Hyde KD (2011) Revision of lignicolous Tubeufiaceae based on morphological reexamination and phylogenetic analysis. Fungal Divers 51:63–102
Boonmee S, Rossman AY, Liu JK, Li WJ, Dai DQ, Bhat JD, Jones EBG, McKenzie EHC, Xu JC, Hyde KD (2014) Tubeufiales, ord. nov., integrating sexual and asexual generic names. Fungal Divers 68:239–298
Coiffard C, Gomez B, Daviero-Gomez V, Dilcher DL (2012) Rise to dominance of angiosperm pioneers in European Cretaceous environments. Proc Natl Acad Sci USA 109:20955–20959
Crous PW, Slippers B, Wingfield MJ, Rheeder J, Marasas WFO, Philips AJL, Alves A, Burgess T, Barber P, Groenewald JZ (2006) Phylogenetic lineages in the Botryosphaeriaceae. Stud Mycol 55:235–253
Dissanayake AJ, Phillips AJL, Li XH, Hyde KD (2016) Botryosphaeriaceae: current status of genera and species. Mycosphere 7:1001–1073
Drummond AJ, Ho SYW, Phillips MJ, Rambaut A (2006) Relaxed phylogenetics and dating with confidence. PLoS Biol 4(5):e88
Drummond AJ, Suchard MA, Xie D, Rambaut A (2012) Bayesian phylogenetics with BEAUti and the BEAST 1.7. Mol Biol Evol 29:1969–1973
Ekanayaka AH, Dissanayake AJ, Jayasiri SC, To-anun C, Jones EBG, Zhao Q, Hyde KD (2016) Aplosporella thailandica; a novel species revealing the sexual-asexual connection in Aplosporellaceae (Botryosphaeriales). Mycosphere 7:440–447
Eriksson OE (1981) The families of bitunicate ascomycetes. Opera Bot 60:1–220
Foster CSP, Ho SYW (2017) Strategies for partitioning clock models in phylogenomic dating: application to the angiosperm evolutionary timescale. Genome Biol Evol 9:2752–2763
Fries EM (1849) Summa Vegetabilium Scandinaveae. Sectio posterior. Typographia Academica, Leipzig, pp 259–572
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl Acids Symp Ser 41:95–98
Hawksworth DL, David JC (1989) Family names: index of fungi supplement. CAB International, Wallingford
Hillis DM, Bull JJ (1993) An empirical test of bootstrapping as a method for assessing confidence in phylogenetic analysis. Syst Biol 42:182–192
Hongsanan S, Maharachchikumbura SSN, Hyde KD, Samarakoon MC, Jeewon R, Zhao Q, Al-Sadi AM, Bahkali AH (2017) An updated phylogeny of Sordariomycetes based on phylogenetic and molecular clock evidence. Fungal Divers 84:25–41
Huang WY, Cai YZ, Surveswaran S, Hyde KD, Corke H, Sun M (2009) Molecular phylogenetic identification of endophytic fungi isolated from three Artemisia species. Fungal Divers 36:69–88
Hyde KD, Maharachchikumbura SSN, Hongsanan S, Samarakoon MC, Lücking R, Pem D, Harishchandra D, Jeewon R, Zhao RL, Xu JC, Liu JK, Al-Sadi AM, Bahkali AH, Elgorban AM (2017) The ranking of fungi: a tribute to David L. Hawksworth on his 70th birthday. Fungal Divers 84:1–23
Liu JK, Phookamsak R, Doilom M, Wikee S, Li YM, Ariyawansha HA, Boonmee S, Chomnunti P, Dai DQ, Bhat DJ, Romero AI, Zhuang WY, Monkai J, Jones EBG, Chukeatirote E, Ko TWK, Zhao YC, Wang Y, Hyde KD (2012) Towards a natural classification of Botryosphaeriales. Fungal Divers 57:149–210
Liu NG, Ariyawansa HA, Hyde KD, Maharachchikumbura SSN, Zhao RL, Phillips AJL, Jayawardena RS, Thambugala KM, Dissanayake AJ, Wijayawardene NN, Liu JK, Liu ZY, Jeewon R, Jones EBG, Jumpathong J (2016) Perspectives into the value of genera, families and orders in classification. Mycosphere 7:1649–1668
Liu J-K, Hyde KD, Jeewon R, Phillips AJL, Maharachchikumbura SSN, Ryberg M, Liu ZY, Zhao Q (2017) Ranking higher taxa using divergence times: a case study in Dothideomycetes. Fungal Divers 84:75–99
Luttrell ES (1955) The ascostromatic Ascomycetes. Mycologia 47:511–532
Luttrell ES (1973) Loculoascomycetes. In: GC Ainsworth, FK Sparrow, and AS Sussman (eds) The fungi. A taxonomic review with keys: 135–219, vol IV part A. Academic Press, New York
McElwain JC, Punyasena SW (2007) Mass extinction events and the plant fossil record. Trends Ecol Evol 22(10):548–557
Miller JH (1928) Biologic studies in the Sphaeriales—I. Mycologia 20:187–213
Minnis AM, Kennedy AH, Grenier DB, Palm ME, Rossman AY (2012) Phylogeny and taxonomic revision of the Planistromellaceae including its coelomycetous anamorphs: contributions towards a monograph of the genus Kellermania. Persoonia 29:11–28
Monkai J, Liu JK, Boonmee S, Chomnunti P, Chukeatirote E, Jones EBG, Wang Y, Hyde KD (2013) Planistromellaceae (Botryosphaeriales). Cryptogamie Mycol 34:45–77
Nylander JAA (2004) MrModeltest v2.2. Program distributed by the author: 2. Evolutionary Biology Centre, Uppsala University, pp 1–2
Phillips AJL, Alves A (2009) Taxonomy, phylogeny, and epitypification of Melanops tulasnei, the type species of Melanops. Fungal Divers 38:155–166
Phillips AJL, Alves A, Pennycook SR, Johnston PR, Ramaley A, Akulov A, Crous P (2008) Resolving the phylogenetic and taxonomic status of dark-spored teleomorph genera in the Botryosphaeriaceae. Persoonia 21:29–55
Phillips AJL, Alves A, Abdollahzadeh J, Slippers B, Wingfield MJ, Groenewald JZ, Crous PW (2013) The Botryosphaeriaceae: genera and species known from culture. Stud Mycol 76:51–167
Ramaley A (1993) New fungi from Yucca: Planistromella yuccifoliorum, gen. et sp. nov., its anamorph, Kellermania yuccifoliorum, sp. nov., and Planistromella uniseptata, sp. nov., the teleomorph of Kellermania yuccigena. Mycotaxon 47:259–274
Rambaut A (2009) FigTree 1.2.2. http://tree.bio.ed.ac.uk/software/figtree/
Rambaut A, Suchard M, Drummond AJ (2013) Tracer 1.6 http://tree.bio.ed.ac.uk/software/tracer/
Rojas EI, Herre EA, Mejía LC, Arnold AE, Chaverri P, Samuels GJ (2008) Endomelanconiopsis, a new anamorph genus in the Botryosphaericeae. Mycologia 100:760–775
Schoch CL, Shoemaker RA, Seifert KA, Hambleton S, Spatafora JW, Crous PW (2006) A multigene phylogeny of the Dothideomycetes using four nuclear loci. Mycologia 98:1041–1052
Seaver FJ (1922) Phyllostictaceae. North American Flora 6:3–84
Sivanesan A (1984) The bitunicate Ascomycetes and their anamorphs. J Cramer, Vaduz, Liechtenstein
Slippers B, Crous PW, Denman S, Coutinho TA, Wingfield BD, Wingfield MJ (2004) Combined multiple gene genealogies and phenotypic characters differentiate several species previously identified as Botryosphaeria dothidea. Mycologia 96:83–101
Slippers B, Boissin E, Phillips AJL, Groenewald JZ, Lombard L, Wingfield MJ, Postma A, Burgess T, Crous PW (2013) Phylogenetic lineages in the Botryosphaeriales: a systematic and evolutionary framework. Stud Mycol 76:31–49
Stamatakis A, Hoover P, Rougemont J (2008) A rapid bootstrap algorithm for the RAxML web servers. Syst Biol 57:758–771
Swofford DL (2003) PAUP* 4.0b10: phylogenetic analysis using parsimony (*and other methods). Sinauer Associates, Sunderland
Theissen F, Sydow H (1918) Vorentwürfe zu den Pseudosphaeriales. Ann Mycol 16:1–34
Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The Clustal_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 25:4876–4882
Vajda V, McLoughlin S (2004) Fungal proliferation at the Cretaceous-Tertiary boundary. Science 303:1489
Vajda V, Raine JI, Hollis CJ (2001) Indication of global deforestation at the Cretaceous-Tertiary boundary by New Zealand fern spike. Science 294:1700–1702
von Arx JA, Müller E (1975) A re-evaluation of the bitunicate ascomycetes with keys to families and genera. Stud Mycol 9:1–159
Wijayawardene NN, Hyde KD, Lumbsch T, Liu JK, Maharachchikumbura SSN, Ekanayaka AH, Tian Q, Phookamsak R (2018) Outline of Ascomycota—2017. Fungal Divers 88:167–263
Wikee S, Udayanga D, Crous PW, Chukeatirote E, McKenzie EHC, Bahkali AH, Dai DQ, Hyde KD (2011) Phyllosticta—an overview of current status of species recognition. Fungal Divers 51:43–61
Wikee S, Lombard L, Nakashima C, Motohashi K, Chukeatirote E, Cheewangkoon R, McKenzie EHC, Hyde KD, Crous PW (2013a) A phylogenetic re-evaluation of Phyllosticta (Botryosphaeriales). Stud Mycol 76:1–29
Wikee S, Lombard L, Crous PW, Nakashima C, Motohashi K, Chukeatirote E, Alias SA, McKenzie EHC, Hyde KD (2013b) Phyllosticta capitalensis, a widespread endophyte of plants. Fungal Divers 60:91–105
Wilf P, Johnson KR (2004) Land plant extinction at the end of the Cretaceous: a quantitative analysis of the North Dakota megafloral record. Paleobiology 30:347–368
Wyka SA, Broders KD (2016) The new family Septorioideaceae, within the Botryosphaeriales and Septorioides strobi as a new species associated with needle defoliation of Pinus strobus in the United States. Fungal Biol 120:1030–1040
Yang T, Groenewald JZ, Cheewangkoon R, Jami F, Abdollahzadeh J, Lombard L, Crous PW (2017) Families, genera, and species of Botryosphaeriales. Fungal Biol 121:322–346
Acknowledgements
Alan JL Phillips acknowledges the support from Biosystems and Integrative Sciences Institute (BioISI, FCT/UID/Multi/04046/2013). Artur Alves acknowledges financial support by European Funds (ERDF) through COMPETE and by National Funds through the Portuguese Foundation for Science and Technology (FCT) to research unit CESAM (UID/AMB/50017/2013 – POCI-01-0145-FEDER-007638). Jian-Kui Liu thanks the National Natural Science Foundation of China (NSFC 31600032) and Science and Technology Foundation of Guizhou Province (LH [2015]7061). KD Hyde acknowledges the support of the Thailand Research Fund grant no RSA5980068 entitled “Biodiversity, phylogeny and role of fungal endophytes on above parts of Rhizophora apiculata and Nypa fruticans”.We thank Anusha Ekanayaka, Saowanee Wikee and Jutamart Monkai for providing photographs of many of the families included in this paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Phillips, A.J.L., Hyde, K.D., Alves, A. et al. Families in Botryosphaeriales: a phylogenetic, morphological and evolutionary perspective. Fungal Diversity 94, 1–22 (2019). https://doi.org/10.1007/s13225-018-0416-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13225-018-0416-6