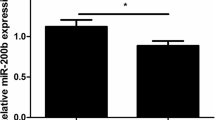
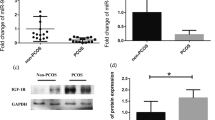
Abstract
Polycystic ovary syndrome (PCOS) is a hormonal disorder common among women of reproductive age. Although much is understood concerning the pathology of PCOS, further investigation into the influence of microribonucleic acids (miRNAs) on the proliferation of ovarian granulosa cells (GCs) is needed. This study investigated the role of specific miRNAs in ovarian dysfunction of PCOS and its effect on the proliferation of GCs. Initially, miRNA profiling was performed on the ovarian cortexes of 15 rats in which PCOS had been induced and 15 rats without PCOS (non-PCOS). This mechanical study was performed on ovarian GCs extracted from human chorionic gonadotrophin (hCG)-induced rats. Insulin was used to treat GCs to establish the PCOS cell model. Increased Equus caballus mir-9119 expression was observed and confirmed in the insulin-induced model of PCOS in GCs (GC-PCOS) as well as in the hCG-induced rats when compared to non-PCOS rats and cells. Observation and confirmation were carried out through both miRNA array and quantitative PCR. In contrast, downregulation of the nuclear factor kappa B (NFκB) p65 was observed in the PCOS cell model. Additionally, annexin V, FITC, and propidium iodide flow cytometry showed overexpression of miR-9119-induced apoptosis. In this study, we revealed that miR-9119 inhibition regulates p65 expression levels in insulin-treated GCs by binding to the 3′-untranslated of p65. Additionally, regulation of p65 expression was positively correlated with the expression of the double-stranded RNA endoribonuclease DICER. Moreover, RNA silencing/overexpression of p65 affected the functional role of miR-9119. In conclusion, GCs of PCOS, the expression of miR-9119, and targeted NFκB/p65-DICER axis are upregulated in order to maintain cell viability and prevent apoptosis, thereby promoting Anti-Müllerian hormone production in GCs. This study may provide a new understanding of the mechanism of GC dysfunction.








Similar content being viewed by others
References
Imbar T, Eisenberg I (2014) Regulatory role of microRNAs in ovarian function. Fertil Steril 101:1524–1530
Baley J, Li J (2012) MicroRNAs and ovarian function. J Ovarian Res 5:8
Azziz R, Sanchez LA, Knochenhauer ES et al (2004) Androgen excess in women: experience with over 1000 consecutive patients. J Clin Endocrinol Metab 89:453–462
Azziz R, Carmina E, Dewailly D et al (2006) Positions statement: criteria for defining polycystic ovary syndrome as a predominantly hyperandrogenic syndrome: an Androgen Excess Society guideline. J Clin Endocrinol Metab 91:4237–4245
Franks S, McCarthy MI, Hardy K (2006) Development of polycystic ovary syndrome: involvement of genetic and environmental factors. Int J Androl 29: 278–85; (discussion 286–290).
Fujii T, Shimada K, Nakai T et al (2018) MicroRNAs in smoking-related carcinogenesis: biomarkers, functions, and therapy. J Clin Med 7:98
Hayes J, Peruzzi PP, Lawler S (2014) MicroRNAs in cancer: biomarkers, functions and therapy. Trends Mol Med 20:460–469
Valadi H, Ekström K, Bossios A et al (2007) Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 9:654
Mitchell PS, Parkin RK, Kroh EM et al (2008) Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci 105:10513–10518
Murri M, Insenser M, Fernández-Durán E et al (2013) Effects of polycystic ovary syndrome (PCOS), sex hormones, and obesity on circulating miRNA-21, miRNA-27b, miRNA-103, and miRNA-155 expression. J Clin Endocrinol Metab 98:E1835–E1844
Salimi-Asl M, Mozdarani H, Kadivar M (2016) Up-regulation of miR-21 and 146a expression and increased DNA damage frequency in a mouse model of polycystic ovary syndrome (PCOS). BioImpacts: BI 6: 85.
Hossain MM, Cao M, Wang Q et al (2013) Altered expression of miRNAs in a dihydrotestosterone-induced rat PCOS model. J Ovarian Res 6:1
Guan Y, Yao H, Wang J et al (2015) NF-κB-DICER-miRs axis regulates TNF-α expression in responses to endotoxin stress. Int J Biol Sci 11:1257
Lei L, Jin S, Gonzalez G et al (2010) The regulatory role of Dicer in folliculogenesis in mice. Mol Cell Endocrinol 315:63–73
Stepto NK, Cassar S, Joham AE et al (2013) Women with polycystic ovary syndrome have intrinsic insulin resistance on euglycaemic-hyperinsulaemic clamp. Hum Reprod 28:777–784
He T, Sun Y, Zhang Y et al (2019) MicroRNA-200b and microRNA-200c are up-regulated in PCOS granulosa cell and inhibit KGN cell proliferation via targeting PTEN. Reprod Biol Endocrinol 17:1–8
Xia Y, Shen S, Verma IM (2014) NF-kappaB, an active player in human cancers. Cancer Immunol Res 2:823–830
Gerondakis S, Grumont R, Gugasyan R et al (2006) Unravelling the complexities of the NF-kappaB signalling pathway using mouse knockout and transgenic models. Oncogene 25:6781–6799
Nguyen DP, Li J, Yadav SS et al (2014) Recent insights into NF-kappaB signalling pathways and the link between inflammation and prostate cancer. BJU Int 114:168–176
Gasparini C, Celeghini C, Monasta L et al (2014) NF-kappaB pathways in hematological malignancies. Cell Mol Life Sci 71:2083–2102
Arlt A, Muerkoster SS, Schafer H (2013) Targeting apoptosis pathways in pancreatic cancer. Cancer Lett 332:346–358
Gyrd-Hansen M, Meier P (2010) IAPs: from caspase inhibitors to modulators of NF-κB, inflammation and cancer. Nat Rev Cancer 10:561
Albert S, Baldwin J (1996) THE NF-κB AND IκB PROTEINS: New Discoveries and Insights. Annu Rev Immunol 14:649–681
Carmell MA, Hannon GJ (2004) RNase III enzymes and the initiation of gene silencing. Nat Struct Mol Biol 11:214
Jin Z, Xie T (2007) Dcr-1 maintains Drosophila ovarian stem cells. Curr Biol 17:539–544
Sun R, Zhang Y, Lv Q et al (2011) Toll-like receptor 3 (TLR3) induces apoptosis via death receptors and mitochondria by up-regulating the transactivating p63 isoform α (TAP63α). J Biol Chem 286:15918–15928
Funding
This study was funded by the National Science Foundation of China (Grant Numbers 81471522; 81671536 and 81871223), the Nature Science Foundation of Guangdong Province of China (Grant Number 2014A030313482), and the Guangdong Provincial Science and Technology Project (Grant Number 2016A020218015).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Every procedure was approved by the Animal Care and Use Committee of the First Affiliated Hospital of Shantou University Medical College.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ding, Y., He, P. & Li, Z. MicroRNA-9119 regulates cell viability of granulosa cells in polycystic ovarian syndrome via mediating Dicer expression. Mol Cell Biochem 465, 187–197 (2020). https://doi.org/10.1007/s11010-019-03678-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-019-03678-6