Abstract
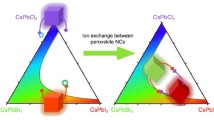
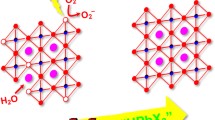
The unique structure of zero-dimensional (0D) perovskite-analogues has attracted a great amount of research interest in recent years. To date, the current compositional library of 0D perovskites is largely limited to the lead-based Cs4PbX6 (X = Cl, Br, and I) systems. In this work, we report a new synthesis of lead-free 0D Cs3BiX6 (X = Cl, Br) perovskite-analogue nanocrystals (NCs) with a uniform cubic shape. We observe a broad photoluminescence peak centered at 390 nm for the 0D Cs3BiCl6 NCs at low temperatures. This feature originates from a self-trapped exciton mechanism. In situ thermal stability studies show that Cs3BiX6 NCs remain stable upon heating up to 200 °C without crystal structural degradation. Moreover, we demonstrate that the Cs3BiX6 NCs can transform into other bismuth-based perovskite-analogues via facile anion exchange or metal ion insertion reactions. Our study presented here offers the opportunity for further understanding of the structure-property relationship of 0D perovskite-analogue materials, leading toward their future optoelectronic applications.

Similar content being viewed by others
References
Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J. Am. Chem. Soc.2009, 131, 6050–6051.
Protesescu, L.; Yakunin, S.; Bodnarchuk, M. I.; Krieg, F.; Caputo, R.; Hendon, C. H.; Yang, R. X.; Walsh, A.; Kovalenko, M. V. Nanocrystals of cesium lead halide perovskites (CsPbX3, X = Cl, Br, and I): Novel optoelectronic materials showing bright emission with wide color gamut. Nano Lett.2015, 15, 3692–3696.
Akkerman, Q. A.; D’Innocenzo, V.; Accornero, S.; Scarpellini, A.; Petrozza, A.; Prato, M.; Manna, L. Tuning the optical properties of cesium lead halide perovskite nanocrystals by anion exchange reactions. J. Am. Chem. Soc.2015, 137, 10276–10281.
Bekenstein, Y.; Koscher, B. A.; Eaton, S. W.; Yang, P. D.; Alivisatos, A. P. Highly luminescent colloidal nanoplates of perovskite cesium lead halide and their oriented assemblies. J. Am. Chem. Soc.2015, 137, 16008–16011.
Nagaoka, Y.; Hills-Kimball, K.; Tan, R.; Li, R. P.; Wang, Z. W.; Chen, O. Nanocube superlattices of cesium lead bromide perovskites and pressure-induced phase transformations at atomic and mesoscale levels. Adv. Mater.2017, 29, 1606666.
Liu, Z. K.; Bekenstein, Y.; Ye, X. C.; Nguyen, S. C.; Swabeck, J.; Zhang, D. D.; Lee, S. T.; Yang, P. D.; Ma, W. L.; Alivisatos, A. P. Ligand mediated transformation of cesium lead bromide perovskite nanocrystals to lead depleted Cs4PbBr6 nanocrystals. J. Am. Chem. Soc.2017, 139, 5309–5312.
Cai, T.; Yang, H. J.; Hills-Kimball, K.; Song, J. P.; Zhu, H.; Hofman, E.; Zheng, W. W.; Rubenstein, B. M.; Chen, O. Synthesis of all-inorganic Cd-doped CsPbCl3 perovskite nanocrystals with dualwavelength emission. J. Phys. Chem. Lett.2018, 9, 7079–7084.
Zhu, H. M.; Fu, Y. P.; Meng, F.; Wu, X. X.; Gong, Z. Z.; Ding, Q.; Gustafsson, M. V.; Trinh, M. T.; Jin, S.; Zhu, X. Y. Lead halide perovskite nanowire lasers with low lasing thresholds and high quality factors. Nat. Mater.2015, 14, 636–642.
Que, M. D.; Dai, Z. H.; Yang, H. J.; Zhu, H.; Zong, Y. X.; Que, W. X.; Padture, N. P.; Zhou, Y. Y.; Chen, O. Quantum-dot-induced cesium-rich surface imparts enhanced stability to formamidinium lead iodide perovskite solar cells. ACS Energy Lett.2019, 4, 1970–1975.
Kroupa, D. M.; Roh, J. Y.; Milstein, T. J.; Creutz, S. E.; Gamelin, D. R. Quantum-cutting ytterbium-doped CsPb(Cl1-xBrx)3 perovskite thin films with photoluminescence quantum yields over 190%. ACS Energy Lett.2018, 3, 2390–2395.
Zhang, F.; Zhong, H. Z.; Chen, C.; Wu, X. G.; Hu, X. M.; Huang, H. L.; Han, J. B.; Zou, B. S.; Dong, Y. P. Brightly luminescent and color-tunable colloidal CH3NH3PbX3 (X = Br, I, Cl) quantum dots: Potential alternatives for display technology. ACS Nano2015, 9, 4533–4542.
Zhu, H.; Cai, T.; Que, M. D.; Song, J. P.; Rubenstein, B. M.; Wang, Z. W.; Chen, O. Pressure-induced phase transformation and band-gap engineering of formamidinium lead iodide perovskite nanocrystals. J. Phys. Chem. Lett.2018, 9, 4199–4205.
Chen, Q. S.; Wu, J.; Ou, X. Y.; Huang, B. L.; Almutlaq, J.; Zhumekenov, A. A.; Guan, X. W.; Han, S. Y.; Liang, L. L.; Yi, Z. G. et al. All-inorganic perovskite nanocrystal scintillators. Nature2018, 561, 88–93.
Zhou, Q. C.; Bai, Z. L.; Lu, W. G.; Wang, Y. T.; Zou, B. S.; Zhong, H. Z. In situ fabrication of halide perovskite nanocrystal- embedded polymer composite films with enhanced photoluminescence for display backlights. Adv. Mater.2016, 28, 9163–9168.
Yang, H. J.; Zhang, Y.; Hills-Kimball, K.; Zhou, Y. Y.; Chen, O. Building bridges between halide perovskite nanocrystals and thin-film solar cells. Sustain. Energy Fuels2018, 2, 2381–2397.
Swarnkar, A.; Marshall, A. R.; Sanehira, E. M.; Chernomordik, B. D.; Moore, D. T.; Christians, J. A.; Chakrabarti, T.; Luther, J. M. Quantum dot-induced phase stabilization of alpha-CsPbI3 perovskite for high-efficiency photovoltaics. Science2016, 354, 92–95.
Manser, J. S.; Christians, J. A.; Kamat, P. V. Intriguing optoelectronic properties of metal halide perovskites. Chem. Rev.2016, 116, 12956–13008.
Xiao, Z. W.; Meng, W. W.; Wang, J. B.; Mitzi, D. B.; Yan, Y. F. Searching for promising new perovskite-based photovoltaic absorbers: The importance of electronic dimensionality. Mater. Horiz.2017, 4, 206–216.
Saidaminov, M. I.; Mohammed, O. F.; Bakr, O. M. Low-dimensional-networked metal halide perovskites: The next big thing. ACS Energy Lett.2017, 2, 889–896.
Yin, J.; Maity, P.; De Bastiani, M.; Dursun, I.; Bakr, O. M.; Brédas, J. L.; Mohammed, O. F. Molecular behavior of zero-dimensional perovskites. Sci. Adv.2017, 3, e1701793.
Almutlaq, J.; Yin, J.; Mohammed, O. F.; Bakr, O. M. The benefit and challenges of zero-dimensional perovskites. J. Phys. Chem. Lett.2018, 9, 4131–4138.
Lin, H. R.; Zhou, C. K.; Tian, Y.; Siegrist, T.; Ma, B. W. Low-dimensional organometal halide perovskites. ACS Energy Lett.2018, 3, 54–62.
Akkerman, Q. A.; Park, S.; Radicchi, E.; Nunzi, F.; Mosconi, E.; De Angelis, F.; Brescia, R.; Rastogi, P.; Prato, M.; Manna, L. Nearly monodisperse insulator Cs4PbX6 (X = Cl, Br, I) nanocrystals, their mixed halide compositions, and their transformation into CsPbX3 nanocrystals. Nano Lett.2017, 17, 1924–1930.
Wu, L. Z.; Hu, H. C.; Xu, Y.; Jiang, S.; Chen, M.; Zhong, Q. X.; Yang, D.; Liu, Q. P.; Zhao, Y.; Sun, B. Q. et al. From nonluminescent Cs4PbX6 (X = Cl, Br, I) nanocrystals to highly luminescent CsPbX3 nanocrystals: Water- triggered transformation through a CsX-stripping mechanism. Nano Lett.2017, 17, 5799–5804.
Hu, H. C.; Wu, L. Z.; Tan, Y. S.; Zhong, Q. X.; Chen, M.; Qiu, Y. H.; Yang, D.; Sun, B. Q.; Zhang, Q.; Yin, Y. D. Interfacial synthesis of highly stable CsPbX3/oxide janus nanoparticles. J. Am. Chem. Soc.2018, 140, 406–412.
Fanizza, E.; Cascella, F.; Altamura, D.; Giannini, C.; Panniello, A.; Triggiani, L.; Panzarea, F.; Depalo, N.; Grisorio, R.; Suranna, G. P. et al. Post-synthesis phase and shape evolution of CsPbBr3 colloidal nanocrystals: The role of ligands. Nano Res.2019, 12, 1155–1166.
Saidaminov, M. I.; Almutlaq, J.; Sarmah, S.; Dursun, I.; Zhumekenov, A. A.; Begum, R.; Pan, J.; Cho, N.; Mohammed, O. F.; Bakr, O. M. Pure Cs4PbBr6: Highly luminescent zero-dimensional perovskite solids. ACS Energy Lett.2016, 1, 840–845.
Chen, D. Q.; Wan, Z. Y.; Chen, X.; Yuan, Y. J.; Zhong, J. S. Large-scale room-temperature synthesis and optical properties of perovskite-related Cs4PbBr6 fluorophores. J. Mater. Chem. C2016, 4, 10646–10653.
Babayigit, A.; Ethirajan, A.; Muller, M.; Conings, B. Toxicity of organometal halide perovskite solar cells. Nat. Mater.2016, 15, 247–251.
Jellicoe, T. C.; Richter, J. M.; Glass, H. F. J.; Tabachnyk, M.; Brady, R.; Dutton, S. E.; Rao, A.; Friend, R. H.; Credgington, D.; Greenham, N. C. et al. Synthesis and optical properties of lead-free cesium tin halide perovskite nanocrystals. J. Am. Chem. Soc.2016, 138, 2941–2944.
Vargas, B.; Ramos, E.; Pérez-Gutiérrez, E.; Alonso, J. C.; Solis-Ibarra, D. A direct bandgap copper-antimony halide perovskite. J. Am. Chem. Soc.2017, 139, 9116–9119.
Luo, J. J.; Wang, X. M.; Li, S. R.; Liu, J.; Guo, Y. M.; Niu, G. D.; Yao, L.; Fu, Y. H.; Gao, L.; Dong, Q. S. et al. Efficient and stable emission of warm-white light from lead-free halide double perovskites. Nature2018, 563, 541–545.
Ju, M. G.; Dai, J.; Ma, L.; Zeng, X. C. Lead-free mixed tin and germanium perovskites for photovoltaic application. J. Am. Chem. Soc.2017, 139, 8038–8043.
Slavney, A. H.; Hu, T.; Lindenberg, A. M.; Karunadasa, H. I. A bismuth-halide double perovskite with long carrier recombination lifetime for photovoltaic applications. J. Am. Chem. Soc.2016, 138, 2138–2141.
Pan, W. C.; Wu, H. D.; Luo, J. J.; Deng, Z. Z.; Ge, C.; Chen, C.; Jiang, X. W.; Yin, W. J.; Niu, G. D.; Zhu, L. J. et al. Cs2AgBiBr6 single-crystal X-ray detectors with a low detection limit. Nat. Photonics2017, 11, 726–732.
Li, Q.; Wang, Y. G.; Pan, W. C.; Yang, W. G.; Zou, B.; Tang, J.; Quan, Z. W. High-pressure band-gap engineering in lead-free Cs2AgBiBr6 double perovskite. Angew. Chem., Int. Ed.2017, 56, 15969–15973.
Chen, N.; Cai, T.; Li, W. H.; Hills-Kimball, K.; Yang, H. J.; Que, M. D.; Nagaoka, Y.; Liu, Z. Y.; Yang, D.; Dong, A. G. et al. Yb- and Mn-doped lead-free double perovskite Cs2AgBiX6 (X = Cl-, Br-) nanocrystals. ACS Appl. Mater. Interfaces2019, 11, 16855–16863.
Bekenstein, Y.; Dahl, J. C.; Huang, J. M.; Osowiecki, W. T.; Swabeck, J. K.; Chan, E. M.; Yang, P. D.; Alivisatos, A. P. The making and breaking of lead-free double perovskite nanocrystals of cesium silver-bismuth halide compositions. Nano Lett.2018, 18, 3502–3508.
Lyu, M.; Yun, J. H.; Cai, M. L.; Jiao, Y. L.; Bernhardt, P. V.; Zhang, M.; Wang, Q.; Du, A. J.; Wang, H. X.; Liu, G. et al. Organic-inorganic bismuth (III)-based material: A lead-free, air-stable and solutionprocessable light-absorber beyond organolead perovskites. Nano Res.2016, 9, 692–702.
Sun, J.; Yang, J.; Lee, J. I.; Cho, J. H.; Kang, M. S. Lead- free perovskite nanocrystals for light-emitting devices. J. Phys. Chem. Lett.2018, 9, 1573–1583.
Li, M. Q.; Hu, Y. Q.; Bi, L. Y.; Zhang, H. L.; Wang, Y. Y.; Zheng, Y. Z. Structure tunable organic-inorganic bismuth halides for an enhanced two-dimensional lead-free light-harvesting material. Chem. Mater.2017, 29, 5463–5467.
Yang, B.; Chen, J. S.; Hong, F.; Mao, X.; Zheng, K. B.; Yang, S. Q.; Li, Y. J.; Pullerits, T.; Deng, W. Q.; Han, K. L. Lead-free, air-stable all-inorganic cesium bismuth halide perovskite nanocrystals. Angew. Chem., Int. Ed.2017, 56, 12471–12475.
Leng, M. Y.; Yang, Y.; Zeng, K.; Chen, Z. W.; Tan, Z. F.; Li, S. R.; Li, J. H.; Xu, B.; Li, D. B.; Hautzinger, M. P. et al. All-inorganic bismuth-based perovskite quantum dots with bright blue photoluminescence and excellent stability. Adv. Funct. Mater.2018, 28, 1704446.
Qi, Z. Y.; Fu, X. W.; Yang, T. F.; Li, D.; Fan, P.; Li, H. L.; Jiang, F.; Li, L. H.; Luo, Z. Y.; Zhuang, X. J. et al. Highly stable lead-free Cs3Bi2I9 perovskite nanoplates for photodetection applications. Nano Res.2019, 12, 1894–1899.
Tong, X. W.; Kong, W. Y.; Wang, Y. Y.; Zhu, J. M.; Luo, L. B.; Wang, Z. H. High-performance red-light photodetector based on lead-free bismuth halide perovskite film. ACS Appl. Mater. Interfaces2017, 9, 18977–18985.
Leng, M. Y.; Chen, Z. W.; Yang, Y.; Li, Z.; Zeng, K.; Li, K. H.; Niu, G. D.; He, Y. S.; Zhou, Q. C.; Tang, J. Lead-free, blue emitting bismuth halide perovskite quantum dots. Angew. Chem., Int. Ed.2016, 55, 15012–15016.
Lou, Y. B.; Fang, M. Y.; Chen, J. X.; Zhao, Y. X. Formation of highly luminescent cesium bismuth halide perovskite quantum dots tuned by anion exchange. Chem. Commun.2018, 54, 3779–3782.
Park, B. W.; Philippe, B.; Zhang, X. L.; Rensmo, H.; Boschloo, G.; Johansson, E. M. J. Bismuth based hybrid perovskites A3Bi2I9 (A: Methylammonium or cesium) for solar cell application. Adv. Mater.2015, 27, 6806–6813.
Zhang, X.; Wu, G.; Gu, Z.; Guo, B.; Liu, W.; Yang, S.; Ye, T.; Chen, C.; Tu, W.; Chen, H. Active-layer evolution and efficiency improvement of (CH3NH3)3Bi2I9-based solar cell on TiO2-deposited ITO substrate. Nano Res.2016, 9, 2921–2930.
Shin, J.; Kim, M.; Jung, S.; Kim, C. S.; Park, J.; Song, A.; Chung, K. B.; Jin, S. H.; Lee, J. H.; Song, M. Enhanced efficiency in lead-free bismuth iodide with post treatment based on a hole-conductor-free perovskite solar cell. Nano Res.2018, 11, 6283–6293.
Bai, F.; Hu, Y. H.; Hu, Y. Q.; Qiu, T.; Miao, X. L.; Zhang, S. F. Lead-free, air-stable ultrathin Cs3Bi2I9 perovskite nanosheets for solar cells. Sol. Energy Mater. Sol. Cells2018, 184, 15–21.
Shimizu, M.; Koshimizu, M.; Fujimoto, Y.; Yanagida, T.; Ono, S.; Asai, K. Luminescence and scintillation properties of Cs3BiCl6 crystals. Opt. Mater.2016, 61, 115–118.
Tang, Y. Y.; Liang, M. L.; Chang, B. D.; Sun, H. Y.; Zheng, K. B.; Pullerits, T.; Chi, Q. J. Lead-free double halide perovskite Cs3BiBr6 with well-defined crystal structure and high thermal stability for optoelectronics. J. Mater. Chem. C2019, 7, 3369–3374.
Creutz, S. E.; Liu, H. B.; Kaiser, M. E.; Li, X. S.; Gamelin, D. R. Structural diversity in cesium bismuth halide nanocrystals. Chem. Mater.2019, 31, 4685–4697.
Imran, M.; Caligiuri, V.; Wang, M. J.; Goldoni, L.; Prato, M.; Krahne, R.; De Trizio, L.; Manna, L. Benzoyl halides as alternative precursors for the colloidal synthesis of lead-based halide perovskite nanocrystals. J. Am. Chem. Soc.2018, 140, 2656–2664.
Radhakrishna, S.; Setty, R. S. S. Bismuth centers in alkali halides. Phys. Rev. B1976, 14, 969–976.
Wang, L. L.; Sun, Q.; Liu, Q. Z.; Shi, J. S. Investigation and application of quantitative relationship between sp energy levels of Bi3+ ion and host lattice. J. Solid State Chem.2012, 191, 142–146.
Xue, J. P.; Wang, X. F.; Jeong, J. H.; Yan, X. H. Spectral and energy transfer in Bi3+-Ren+ (n = 2, 3, 4) co-doped phosphors: Extended optical applications. Phys. Chem. Chem. Phys.2018, 20, 11516–11541.
Toyozawa, Y.; Inoue, M. Dynamical Jahn-Teller effect in alkali halide phosphors containing heavy metal ions. J. Phys. Soc. Jpn.1966, 21, 1663–1679.
Hills-Kimball, K.; Nagaoka, Y.; Cao, C.; Chaykovsky, E.; Chen, O. Synthesis of formamidinium lead halide perovskite nanocrystals through solid-liquid-solid cation exchange. J. Mater. Chem. C2017, 5, 5680–5684.
Shannon, R. D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. A1976, 32, 751–767.
Li, Q.; Yin, L. X.; Chen, Z. W.; Deng, K. R.; Luo, S. P.; Zou, B.; Wang, Z. W.; Tang, J.; Quan, Z. W. High pressure structural and optical properties of two-dimensional hybrid halide perovskite (CH3NH3)3Bi2Br9. Inorg. Chem.2019, 58, 1621–1626.
Leng, M. Y.; Yang, Y.; Chen, Z. W.; Gao, W. R.; Zhang, J.; Niu, G. D.; Li, D. B.; Song, H. S.; Zhang, J. B.; Jin, S. et al. Surface passivation of bismuth-based perovskite variant quantum dots to achieve efficient blue emission. Nano Lett.2018, 18, 6076–6083.
Ng, T. W.; Chan, C. Y.; Lo, M. F.; Guan, Z. Q.; Lee, C. S. Formation chemistry of perovskites with mixed iodide/chloride content and the implications on charge transport properties. J. Mater. Chem. A2015, 3, 9081–9085.
Wu, N. Q.; Fu, L.; Su, M.; Aslam, M.; Wong, K. C.; Dravid, V. P. Interaction of fatty acid monolayers with cobalt nanoparticles. Nano Lett.2004, 4, 383–386.
Nagaoka, Y.; Tan, R.; Li, R. P.; Zhu, H.; Eggert, D.; Wu, Y. A.; Liu, Y. Z.; Wang, Z. W.; Chen, O. Superstructures generated from truncated tetrahedral quantum dots. Nature2018, 561, 378–382.
Chen, O.; Yang, Y. A.; Wang, T.; Wu, H. M.; Niu, C. G.; Yang, J. H.; Cao, Y. C. Surface-functionalization-dependent optical properties of II-VI semiconductor nanocrystals. J. Am. Chem. Soc.2011, 133, 17504–17512.
Sun, S. B.; Yuan, D.; Xu, Y.; Wang, A. F.; Deng, Z. T. Ligand-mediated synthesis of shape-controlled cesium lead halide perovskite nanocrystals via reprecipitation process at room temperature. ACS Nano2016, 10, 3648–3657.
McCall, K. M.; Stoumpos, C. C.; Kontsevoi, O. Y.; Alexander, G. C. B.; Wessels, B. W.; Kanatzidis, M. G. From 0D Cs3Bi2I9 to 2D Cs3Bi2I6Cl3: Dimensional expansion induces a direct band gap but enhances electron-phonon coupling. Chem. Mater.2019, 31, 2644–2650.
Benin, B. M.; Dirin, D. N.; Morad, V.; Wörle, M.; Yakunin, S.; Rainò, G.; Nazarenko, O.; Fischer, M.; Infante, I.; Kovalenko, M. V. Highly emissive self-trapped excitons in fully inorganic zerodimensional tin halides. Angew. Chem., Int. Ed.2018, 57, 11329–11333.
Thirumal, K.; Chong, W. K.; Xie, W.; Ganguly, R.; Muduli, S. K.; Sherburne, M.; Asta, M.; Mhaisalkar, S.; Sum, T. C.; Soo, H. S. et al. Morphology- independent stable white-light emission from selfassembled two-dimensional perovskites driven by strong exciton- phonon coupling to the organic framework. Chem. Mater.2017, 29, 3947–3953.
McCall, K. M.; Stoumpos, C. C.; Kostina, S. S.; Kanatzidis, M. G.; Wessels, B. W. Strong electron-phonon coupling and self-trapped excitons in the defect halide perovskites A3M2I9 (A = Cs, Rb; M = Bi, Sb). Chem. Mater.2017, 29, 4129–4145.
Ma, Z. W.; Liu, Z.; Lu, S. Y.; Wang, L. R.; Feng, X. L.; Yang, D. W.; Wang, K.; Xiao, G. J.; Zhang, L. J.; Redfern, S. A. T. et al. Pressureinduced emission of cesium lead halide perovskite nanocrystals. Nat. Commun.2018, 9, 4506.
Shi, Y.; Ma, Z. W.; Zhao, D. L.; Chen, Y. P.; Cao, Y.; Wang, K.; Xiao, G. J.; Zou, B. Pressure-induced emission (PIE) of one-dimensional organic tin bromide perovskites. J. Am. Chem. Soc.2019, 141, 6504–6508.
Mao, L. L.; Wu, Y. L.; Stoumpos, C. C.; Wasielewski, M. R.; Kanatzidis, M. G. White-light emission and structural distortion in new corrugated two-dimensional lead bromide perovskites. J. Am. Chem. Soc.2017, 139, 5210–5215.
Dohner, E. R.; Jaffe, A.; Bradshaw, L. R.; Karunadasa, H. I. Intrinsic white-light emission from layered hybrid perovskites. J. Am. Chem. Soc.2014, 136, 13154–13157.
Li, S. R.; Luo, J. J.; Liu, J.; Tang, J. Self-trapped excitons in allinorganic halide perovskites: Fundamentals, status, and potential applications. J. Phys. Chem. Lett.2019, 10, 1999–2007.
Fukuda, A. Jahn-teller effect on the structure of the emission produced by excitation in the A band of KI: Tl-type phosphors. Two kinds of minima on the Γ4−(3T1u) adiabatic potential-energy surface. Phys. Rev. B1970, 1, 4161–4178.
Pelle, F.; Jacquier, B.; Denis, J. P.; Blanzat, B. Optical properties of Cs2NaBiCl6. J. Lumin.1978, 17, 61–72.
Kang, J. G.; Yoon, H. M.; Chun, G. M.; Kim, Y. D.; Tsuboi, T. Spectroscopic studies of Bi3+ colour centres in KCl single crystals. J. Phys.: Condens. Matter1994, 6, 2101–2116.
Nedelcu, G.; Protesescu, L.; Yakunin, S.; Bodnarchuk, M. I.; Grotevent, M. J.; Kovalenko, M. V. Fast anion- exchange in highly luminescent nanocrystals of cesium lead halide perovskites (CsPbX3, X = Cl, Br, I). Nano Lett.2015, 15, 5635–5640.
Creutz, S. E.; Crites, E. N.; De Siena, M. C.; Gamelin, D. R. Anion exchange in cesium lead halide perovskite nanocrystals and thin films using trimethylsilyl halide reagents. Chem. Mater.2018, 30, 4887–4891.
Pal, J.; Bhunia, A.; Chakraborty, S.; Manna, S.; Das, S.; Dewan, A.; Datta, S.; Nag, A. Synthesis and optical properties of colloidal M3Bi2I9 (M = Cs, Rb) perovskite nanocrystals. J. Phys. Chem. C2018, 122, 10643–10649.
Zhang, Y. H.; Yin, J.; Parida, M. R.; Ahmed, G. H.; Pan, J.; Bakr, O. M.; Brédas, J. L.; Mohammed, O. F. Direct-indirect nature of the bandgap in lead-free perovskite nanocrystals. J. Phys. Chem. Lett.2017, 8, 3173–3177.
Udayabhaskararao, T.; Houben, L.; Cohen, H.; Menahem, M.; Pinkas, I.; Avram, L.; Wolf, T.; Teitelboim, A.; Leskes, M.; Yaffe, O. et al. A mechanistic study of phase transformation in perovskite nanocrystals driven by ligand passivation. Chem. Mater.2018, 30, 84–93.
Palazon, F.; Urso, C.; De Trizio, L.; Akkerman, Q.; Marras, S.; Locardi, F.; Nelli, I.; Ferretti, M.; Prato, M.; Manna, L. Postsynthesis transformation of insulating Cs4PbBr6 nanocrystals into bright perovskite CsPbBr3 through physical and chemical extraction of CsBr. ACS Energy Lett.2017, 2, 2445–2448.
Nelson, R. D.; Santra, K.; Wang, Y.; Hadi, A.; Petrich, J. W.; Panthani, M. G. Synthesis and optical properties of ordered-vacancy perovskite cesium bismuth halide nanocrystals. Chem. Commun.2018, 54, 3640–3643.
Acknowledgements
O. C. acknowledges the support from Brown University startup funds and the National Science Foundation (OIA-1538893). K. H. -K. is supported by the U.S. Department of Education GAANN research fellowship (P200A150037). TEM, XRD, XPS and Raman measurements were performed at the Electron Microscopy Facility and NanoTools Facility in the Institute for Molecular and Nanoscale Innovation (IMNI) in Brown University. Part of the photoluminescence measurements was performed at Clemson University.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Yang, H., Cai, T., Liu, E. et al. Synthesis and transformation of zero-dimensional Cs3BiX6 (X = Cl, Br) perovskite-analogue nanocrystals. Nano Res. 13, 282–291 (2020). https://doi.org/10.1007/s12274-019-2611-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-019-2611-5