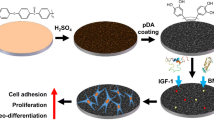
Abstract
Polyether-ether-ketone (PEEK) is becoming a popular component of clinical spinal and orthopedic applications, but its practical use suffers from several limitations. In this study, irregular nano-porous monolayer with differently functional groups was formed on the surface of PEEK through sulfonation and nitrification. The surface characteristics were detected by field-emission scanning electron microscopy, atomic force microscopy, energy-dispersive X-ray spectrometry, water contact angle measurements and Fourier transform infrared spectroscopy. In vitro cellular behaviors were evaluated by cell adhesion, morphological changes, proliferation, alkalinity, phosphatase activity, real-time RT-PCR and western blot analyses. In vivo osseointegration was examined through micro-CT and histological assessments. Our results reveal that the irregular nano-porous of PEEK affect the biological properties. High-temperature hydrothermal NP treatment induced early osteogenic differentiation and early osteogenesis. Modification by sulfonation and nitrification can broaden the use of PEEK in orthopedic and dental applications. This study provides a theoretical basis for the wider clinical application of PEEK.

a To obtain a uniform porous structure, PEEK samples were treated by concentrated sulfuric acid and fuming nitric acid (82–80%) with magnetic stirring sequentially. b Effects of nanopores on biological behavior of bMSCS.





Similar content being viewed by others
References
Wang H, Xu M, Zhang W, Kwok DT, Jiang J, Wu Z et al. Mechanical and biological characteristics of diamond-like carbon coated poly aryl-ether-ether-ketone. Biomaterials. 2010;31:8181–7.
Hieda A, Uemura N, Hashimoto Y, Toda I, Baba S. In vivo bioactivity of porous polyetheretherketone with a foamed surface. Dent Mater J. 2017;36:222.
Kurtz SM, Devine JN. PEEK biomaterials in trauma, orthopedic, and spinal implants. Biomaterials. 2007;28:4845–69.
Wang L, He S, Wu X, Liang S, Mu Z, Wei J et al. Polyetheretherketone/nano-fluorohydroxyapatite composite with antimicrobial activity and osseointegration properties. Biomaterials. 2014;35:6758–75.
Pezzotti G, Oba N, Zhu W, Marin E, Rondinella A, Boschetto F et al. Human osteoblasts grow transitional Si/N apatite in quickly osteointegrated Si3N4 cervical insert. Acta Biomaterialia. 2017;64:411–29.
Gui N, Xu W, Abraham AN, Myers DE, Mayes E, Xia K et al. A comparative study of the effect of submicron porous and smooth ultrafine-grained Ti-20Mo surfaces on osteoblast responses. 2018;106:2020–33.
Alves AC, Thibeaux R, Toptan F, Pinto AMP, Ponthiaux P, David B. Influence of macroporosity on NIH/3T3 adhesion, proliferation, and osteogenic differentiation of MC3T3‐E1 over bio-functionalized highly porous titanium implant material. J Biomed Mater Res Part B Appl Biomater. 2018;107:73–85.
Zhao Y, Wong HM, Wang W, Li P, Xu Z, Chong EYW et al. Cytocompatibility, osseointegration, and bioactivity of three-dimensional porous and nanostructured network on polyetheretherketone. Biomaterials. 2013;34:9264–77.
Torstrick FB, Evans NT, Stevens HY, Gall K, Guldberg RE. Do surface porosity and pore size influence mechanical properties and cellular response to PEEK? Clin Orthop Relat Res®. 2016;474:1–11.
Evans NT, Torstrick FB, Lee CSD, Dupont KM, Safranski DL, Chang WA et al. High strength, surface porous polyether-ether-ketone for load-bearing orthopaedic implants. Acta Biomater. 2015;13:159–67.
Lai M, Cai Y, Hermann CD, Cheng A, Walker M, Olivares-Navarrete R et al. Cell morphology correlates with integrin expression on microrough titanium surfaces. In: Iadr/aadr/cadr General Session and Exhibition. 2013.
Schwarting T, Lechler P, Struewer J, Ambrock M, Frangen TM, Ruchholtz S et al. Bone morphogenetic protein 7 (BMP-7) Influences tendon-bone integration in vitro. PLoS One. 2015;10:e0116833.
Ouyang L, Zhao Y, Jin G, Lu T, Li J, Qiao Y et al. Influence of sulfur content on bone formation and antibacterial ability of sulfonated PEEK. Biomaterials. 2016;83:115.
Tsai JC, Kuo JF, Chen CY. Nafion®/nitrated sulfonated poly(ether ether ketone) membranes for direct methanol fuel cells. J Power Sour. 2009;194:226–33.
Krygowski TM, Oziminski WP. Substituent effects in 1-nitro-4-substituted bicyclo[2.2.2]octane derivatives: inductive or field effects? J Mol Model. 2014;20:2352.
Rosa AL, Kato RB, Castro Raucci LM, Teixeira LN, de Oliveira FS, Bellesini LS et al. Nanotopography drives stem cell fate toward osteoblast differentiation through α1β1 integrin signaling pathway. J Cell Biochem. 2014;115:540–8.
Zhu Y, Zhang K, Zhao R, Ye X, Chen X, Xiao Z et al. Bone regeneration with micro/nano hybrid-structured biphasic calcium phosphate bioceramics at segmental bone defect and the induced immunoregulation of MSCs. Biomaterials. 2017;147:133.
Gittens RA, Olivaresnavarrete R, Schwartz Z, Boyan BD. Implant osseointegration and the role of microroughness and nanostructures: lessons for spine implants. Acta Biomater. 2014;10:3363–71.
Liu Q, Wang W, Zhang L, Zhao L, Song W, Duan X et al. Involvement of N-cadherin/β-catenin interaction in the micro/nanotopography induced indirect mechanotransduction. Biomaterials. 2014;35:6206–18.
Zhao L, Liu L, Wu Z, Zhang Y, Chu PK. Effects of micropitted/nanotubular titania topographies on bone mesenchymal stem cell osteogenic differentiation. Biomaterials. 2012;33:2629.
Malec K, Góralska J, Hubalewskamazgaj M, Głowacz P, Jarosz M, Brzewski P et al. Effects of nanoporous anodic titanium oxide on human adipose derived stem cells. Int J Nanomed. 2016;11:5349–60.
Teo BK, Wong ST, Lim CK, Kung TY, Yap CH, Ramagopal Y et al. Nanotopography modulates mechanotransduction of stem cells and induces differentiation through focal adhesion kinase. Acs Nano. 2013;7:4785–98.
Zhang W, Dong R, Diao S, Du J, Fan Z, Wang F. Differential long noncoding RNA/mRNA expression profiling and functional network analysis during osteogenic differentiation of human bone marrow mesenchymal stem cells. Stem Cell Res Ther. 2017;8:30.
Ffb H, Papenburg B, Vasilevich A, Hulsman M, Zhao Y, Levers M et al. Mining for osteogenic surface topographies: in silico design to in vivo osseo-integration. Biomaterials. 2017;137:49.
Spriano S, Yamaguchi S, Baino F, Ferraris S. A critical review of multifunctional titanium surfaces: New frontiers for improving osseointegration and host response, avoiding bacteria contamination. Acta Biomater. 2018;79:1–22.
Refai AK, Textor M, Brunette DM, Waterfield JD. Effect of titanium surface topography on macrophage activation and secretion of proinflammatory cytokines and chemokines. J Biomed Mater Res Part A. 2004;70:194–205.
Kawai T, Takemoto M, Fujibayashi S, Neo M, Kokubo T. Bone-bonding properties of Ti metal subjected to acid and heat treatments. J Mater Sci Mater Med. 2012;23:2981–92.
Acknowledgements
The authors thank GW and JW of PLA 960th hospital (Jinan, China) for they earnest guidance. This work was supported by grants from National Natural Science Foundation of China (61471384, 81602651, 61701520, 61771290, 61871393). Science and Technology Development Plans of Shandong province (2018GSF118196). Taishan Scholars (tsqn201812137). The Fundamental Research Funds of Shandong University (2019GN091).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest
Ethical statement
In this research all animal procedures were performed in accordance with the standards of the Ethics Committee of Shandong university (NO. GD201701, 02-27-2017).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, Y., Wang, J., He, D. et al. Surface sulfonation and nitrification enhance the biological activity and osteogenesis of polyetheretherketone by forming an irregular nano-porous monolayer. J Mater Sci: Mater Med 31, 11 (2020). https://doi.org/10.1007/s10856-019-6349-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10856-019-6349-0