Abstract
Multilayered and porous sodium-doped graphitic carbon nitride (GCN-Na) was prepared and employed to the solid-phase extraction of Sr(II). The sorbent exhibits high adsorption capacity and excellent selectivity for Sr(II). This is due to its small interplanar stacking distance caused by doping with Na(I) which matches the size of Sr(II) better than blank GCN. An original solid-phase extraction method based on GCN-Na coupled with ICP-OES was established for Sr(II), the calibration plots are linear ranging from 0.05–10 mg·kg−1 with the correlation coefficients (R2) above 0.999, the limits of detection are in the range of 0.57–1.52 μg·kg−1 and the preconcentration factor of 80 is achieved using 48 mL sample. It was successfully applied in the extraction and detection of trace Sr(II) in tap water, rice and sea fish.

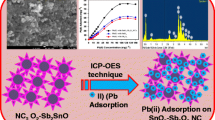
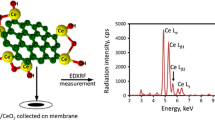
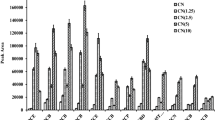
A multilayer porous sodium(I) doped graphitic carbon nitride nanosheet (GCN-Na) was synthesized and exhibited excellent adsorption capability and selectivity for Sr(II).






Similar content being viewed by others
References
Miura T, Minai Y (2017) Radiometric analysis of 90Sr in fish bone ash samples by liquid scintillationcounting after separation by extraction chromatographic resin. J Radioanal Nucl Chem 313:343–351
Pittet PA, Bochud F, Froidevaux P (2019) Determination of 89Sr and 90Sr in fresh cow milk and raw urine using crystalline synthetic tunnel manganese oxides and layered metal sulfides. Anal Chim Acta 1047:267–274
Cerdà V (2019) Automation of radiochemical analysis by flow techniques−a review. Trends Anal Chem 18:352–367
Ayala A, Takagai Y (2018) On-line pseudo-stationary magnetic solid-phase extraction using magnetic cation exchange microparticles and its application to the determination of strontium. J Anal At Spectrom 33:1251–1255
Shao Y, Yang GS, Tazoe H, Ma LL, Yamada M, Xu DD (2018) A review of measurement methodologies and their applications to environmental 90Sr. J Environ Radioact 192:321–333
Hong HJ, Park IS, Ryu T, Jeong HS, Ryu J (2018) Demonstration of seawater strontium (Sr(II)) extraction and enrichment by a biosorption technique through continuous column operation. Ind Eng Chem Res 57:12909–12915
Kavasi N, Sahoo SK, Aono T (2018) Zirconium decontamination factor test on DGA and Sr resin for 90Sr analysis using inorganic mass spectrometry. J Radioanal Nucl Chem 319:1339–1344
Dyer A, Pillinger M, Harjula R, Amin S (2000) Sorption characteristics of radionuclides on synthetic birnessite-type layered manganese oxides. J MaterChem 10:1867–1874
Jia F, Li JF, Wang JL, Sun YL (2017) Removal of strontium ions from simulated radioactive wastewater by vacuum membrane distillation. Ann Nucl Energy 103:363–368
Tomita J, Yamamoto M, Nozaki T, Tanimura Y, Oishi T (2015) Determination of low-level radiostrontium, with emphasis on in situ pre-concentration of Sr from large volume of freshwater sample using powdex resin. J Environ Radioact 146:88–93
Kamaraj R, Vasudevan S (2015) Evaluation of electrocoagulation process for the removal of strontium and cesium from aqueous solution. Chem Eng Res Des 93:522–530
Kong XY, Dang L, Shao XZ, Yin LL, Ji YQ (2018) Rapid method for determination of 90Sr in biological samples by liquid scintillation counting after separation on synthesized column. J Environ Radioact 193-194:15–19
Yi R, Ye G, Wu FC, Lv DC, Chen J (2015) Magnetic solid-phase extraction of strontium using core–shell structured magnetic microspheres impregnated with crown ether receptors: a response surface optimization. J Radioanal Nucl Chem 308:599–608
Liu Y, Meng XG, Luo M, Meng MJ, Ni L, Qiu J, Hu ZY, Liu FF, Zhong GX, Liu ZC, Yan YS (2015) Synthesis of hydrophilic surface ion-imprinted polymer based on graphene oxide for removal of strontium from aqueous solution. J Mater Chem A 3:1287–1297
Lu YQ, Yu JT, Cheng SG (2014) Magnetic composite of Fe3O4 and activated carbon as a adsorbent for separation of trace Sr(II) from radioactive wastewater. J Radioanal Nucl Chem 303:2371–2377
Chen CL, Hu J, Shao DD, Li JX, Wang XK (2009) Adsorption behavior of multiwall carbon nanotube/iron oxide magnetic composites for Ni(II) and Sr(II). J Hazard Mater 164:923–928
Gujar RB, Mohapatra PK, Verboom W (2019) Two novel extraction chromatographic resins containingbenzene-centered tripodal diglycolamide ligands: Actinide uptake,kinetic modeling and isotherm studies. J Chromatogr A 1598:58–66
Hao X, Chen RR, Liu Q, Liu JY, Zhang HS, Yu J, Li ZS, Wang J (2018) A novel U(VI)-imprinted graphitic carbon nitride composite for the selective and efficient removal of U(VI) from simulated seawater. Inorg Chem Front 5:2218–2226
Sun XP, Ha W, Chen J, Qi HY, Shi YP (2016) Advances and applications of graphitic carbon nitride as sorbent in analytical chemistry for sample pretreatment: a review. Trends Anal Chem 84:12–21
Zhang J, Hu SZ, Wang YJ (2014) A convenient method to prepare a novel alkali metal sodium doped carbon nitride photocatalyst with a tunable band structure. RSC Adv 4:62912–62919
Yang SB, Feng XL, Müllen K (2011) Sandwich-like, graphene-based titania nanosheets with high surface area for fast lithium storage. Adv Mater 23:3575–3579
Zhang XL, Zheng C, Guo SS, Li J, Yang HH, Chen G (2014) Turn-on fluorescence sensor for intracellular imaging of glutathione using g-C3N4 nanosheet-MnO2 sandwich nanocomposite. Anal Chem 86:3426–3434
Hu R, Wang XK, Dai SY, Shao DD, Hayat T, Alsaedi A (2015) Application of graphitic carbon nitride for the removal of Pb(II) and aniline from aqueous solutions. Chem Eng J 260:469–477
Zou YD, Wang XX, Ai YJ, Liu YH, Ji YF, Wang HQ, Hayat T, Alsaedi A, Hu WP, Wang XK (2016) β-Cyclodextrin modified graphitic carbon nitride for the removal of pollutants from aqueous solution: experimental and theoretical calculation study. J Mater Chem A 4:14170–14179
Yu HJ, Shi R, Zhao YX, Bian T, Zhao YF, Zhou C, Waterhouse GIN, Wu LZ, Tung CH, Zhang TR (2017) Alkali-assisted synthesis of nitrogen deficient graphitic carbon nitride with tunable band structures for efficient visible-light-driven hydrogen evolution. Adv Mater 29:1605148–1605155
Jiang LB, Yuan XZ, Pan Y, Liang J, Zeng GM, Wu ZB, Wang H (2017) Doping of graphitic carbon nitride for photocatalysis: a review. Appl Catal, B 217:388–406
Li X, Xing JL, Zhang CL, Han B, Zhang YH, Wen T, Leng R, Jiang ZH, Ai YJ, Wang XK (2018) Adsorption of lead on sulfur-doped graphitic carbon nitride nanosheets: experimental and theoretical calculation study. ACS Sustain Chem Eng 6:10606–10615
Liu JL, Zhang YQ, Zhang L, Xie FX, Vasileff A, Qiao SZ (2019) Graphitic carbon nitride (g-C3N4)-derived N-rich graphene with tuneable interlayer distance as a high-rate anode for sodium-ion batteries. Adv Mater 1901261-1901271
Pan YT, Li DD, Jiang HL (2018) Sodium-doped C3N4/MOF heterojunction composites with tunable band structures for photocatalysis: interplay between light harvesting and electron transfer. Chem Eur J 24:18403–18407
Xu J, Long KZ, Wang Y, Xue B, Li YX (2015) Fast and facile preparation of metal-doped g-C3N4 composites for catalytic synthesis of dimethyl carbonate. Appl Catal, A 496:1–8
Wang JC, Cui CX, Li Y, Liu L, Zhang YP, Shi WN (2017) Porous Mn doped g-C3N4 photocatalysts for enhanced synergetic degradation under visible light illumination. J Hazard Mater 339:43–53
Zou XX, Silva R, Goswami A, Asefa T (2015) Cu-doped carbon nitride: bio-inspired synthesis of H2-evolving electrocatalysts using graphitic carbon nitride (g-C3N4) as a host material. Appl SurfSci 357:221–228
Jiang J, Cao SW, Hu CL, Chen CH (2017) A comparison study of alkali metal-doped g-C3N4 for visible-light photocatalytic hydrogen evolution. Chin J Catal 38:1981–1989
Zhang ZL, Li L (2014) Synthesis and characterization of whisker surface imprinted polymer and selective solid-phase extraction of trace Sr(II) from environment aqueous solution. Desalin Water Treat 54:2441–2451
Yin LL, Kong XY, Zhang Y, Ji YQ (2018) Facile synthesis of the magnetic metal organic framework Fe3O4@UiO-66-NH2 for separation of strontium. Biomed Environ Sci 31:483–488
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant 21775153and 21575150) and the National Key R&D Program of China (2017YFF0211100).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts to declare.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOC 19.1 mb)
Rights and permissions
About this article
Cite this article
Kang, JY., Ha, W., Zhang, HX. et al. Sodium(I)-doped graphitic carbon nitride with appropriate interlayer distance as a highly selective sorbent for strontium(II) prior to its determination by ICP-OES. Microchim Acta 187, 76 (2020). https://doi.org/10.1007/s00604-019-4042-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-019-4042-0