Abstract
Hydatidiform mole (HM) is an aberrant human pregnancy characterized by excessive trophoblastic proliferation and abnormal embryonic development. HM has two morphological types, complete (CHM) and partial (PHM), and non-recurrent ones have three genotypic types, androgenetic monospermic, androgenetic dispermic, and triploid dispermic. Most available studies on risk factors predisposing to different types of HM and their malignant transformation mainly suffer from the lack of comprehensive genotypic analysis of large cohorts of molar tissues combined with accurate postmolar hCG follow-up. Moreover, 10–20% of patients with one HM have at least one non-molar miscarriage, which is higher than the frequency of two pregnancy losses in the general population (2–5%), suggesting a common genetic susceptibility to HM and miscarriages. However, the underlying causes of the miscarriages in these patients are unknown. Here, we comprehensively analyzed 204 HM, mostly from patients referred to the Quebec Registry of Trophoblastic Diseases and for which postmolar hCG monitoring is available, and 30 of their non-molar miscarriages. We revisited the risk of maternal age and neoplastic transformation across the different HM genotypic categories and investigated the presence of chromosomal abnormalities in their non-molar miscarriages. We confirm that androgenetic CHM is more prone to gestational trophoblastic neoplasia (GTN) than triploid dispermic PHM, and androgenetic dispermic CHM is more prone to high-risk GTN and choriocarcinoma (CC) than androgenetic monospermic CHM. We also confirm the association between increased maternal age and androgenetic CHM and their malignancies. Most importantly, we demonstrate for the first time that patients with an HM and miscarriages are at higher risk for aneuploid miscarriages [83.3%, 95% confidence interval (CI): 0.653–0.944] than women with sporadic (51.5%, 95% CI: 50.3–52.7%, p value = 0.0003828) or recurrent miscarriages (43.8%, 95% CI: 40.7–47.0%, p value = 0.00002). Our data suggest common genetic female germline defects predisposing to HM and aneuploid non-molar miscarriages in some patients.
Similar content being viewed by others
Introduction
Hydatidiform mole (HM) is a human pregnancy characterized by abnormal embryonic development, hydropic degeneration of chorionic villi, and excessive trophoblastic proliferation. In the past, HM used to be divided into two types, complete HM (CHM) and partial HM (PHM), based on morphological and cytogenetic evaluation [1, 2].
Since the original description of the two morphological entities, different methods have been developed to determine the parental contribution to the molar genomes and led to the conclusions that most CHM is diploid androgenetic monospermic, most PHM is triploid dispermic, and several morphologically diagnosed CHM or PHM are diploid biparental [3]. In the last 10 years, the improvement of existing methods, the emergence of more informative and efficient genotyping methods by multiplexing several markers, and the combined use of several methods have led to more accurate conclusions about molar genotypes. While androgenetic monospermic, androgenetic dispermic, and triploid dispermic genotypes are now believed to be the only genotypic types of sporadic HM, their frequencies were slightly revised as follows. Of androgenetic CHM, 85% are monospermic and 15% are dispermic [4]. Among triploid PHM, 98% are dispermic and 2% are monospermic [4, 5]. However, most diploid biparental conceptions previously diagnosed as HM are now believed to have been misclassified as HM and are indeed diploid biparental aneuploid conceptions that have some morphological features of moles [5,6,7,8]. The only exception to this is recurrent diploid biparental HM from patients with biallelic mutations in NLRP7 [9,10,11], KHDC3L [12,13,14,15], or rarely PADI6 [16], which may have the morphological features of complete and/or partial HM [9] and are sometimes diagnosed as atypical HM [10, 11]. Other very rare types of conceptions that may morphologically mimic HM and be misdiagnosed as CHM or PHM are tetraploid conceptions and triploid digynic conceptions.
The goal of this study is to comprehensively analyze a large cohort of sporadic moles, mostly from the Quebec Registry of Trophoblastic Diseases, with complete follow-up and postmolar hCG monitoring and re-evaluate some of the risk factors for HM in relation to the accurate molar genotypes. In addition, a history of miscarriages is a well-documented risk factor for HM [17,18,19]. However, the causes of miscarriages in women with one HM have remained unknown. To answer this question, we investigated the causes in the non-molar miscarriages of patients with CHM or PHM.
Materials and methods
Patients
Patients with an HM were referred to our laboratory between 2006 and 2017; the majority (184 out of 204) were from the Quebec Trophoblastic Disease Registry (Registre des Maladies Trophoblastiques du Québec, RMTQ, http://www.rmtq.ca/en/) [20] and some were from other collaborators. All recruited patients provided written consent to participate in our study, agreed to a blood draw for genotyping analysis, and agreed for us to retrieve their molar and non-molar products of conception (POCs) from their various histopathology laboratories for research purposes and to have access to their medical files. Our study population was also combined with that of Banet et al. [4] to test for certain associations. This study was approved by the McGill Institutional Review Board (IRB# A01-M07-98 03A).
Histopathological review
Hematoxylin and eosin-stained tissue sections of the POCs were morphologically evaluated independently by two pathologists (K.R. and J.A.) according to standard criteria [1]. For all molar tissues, the diagnosis was revised to take into consideration the integrated data from various methods. GTN diagnosis and staging were performed according to the International Federation of Gynecology and Obstetrics (FIGO) criteria [21]. Choriocarcinoma diagnosis (by K.R.) was based on histopathology and the presence of biphasic proliferation of mononucleate trophoblast and syncytiotrophoblast cells with the absence of chorionic villi and the presence of hemorrhagic areas associated with significant and variable amounts of necrosis.
Parental contribution to the molar tissues
P57KIP2 immunohistochemistry
P57KIP2 immunohistochemistry was performed on 4-μm sections of formalin-fixed paraffin-embedded (FFPE) tissues as previously described [22]. For each POC, the p57KIP2 immunostaining result was interpreted as negative when endometrial and/or extravillous trophoblastic cells (EVT), which serve as an internal positive control, exhibited nuclear p57KIP2 staining but villous stromal and/or cytotrophoblastic cells did not. The result was interpreted as positive when cytotrophoblast and/or villous stromal cells showed nuclear staining of p57KIP2.
Flow cytometry
Flow cytometry was performed on FFPE tissues following Hedley’s protocol [23] with modifications as previously described [24]. Briefly, two 60-µm sections from each FFPE block were deparaffinized with xylene and gradually rehydrated. The proteins were digested in 1 ml of 5 mg/ml pepsin (Sigma-Aldrich, St. Louis, USA) in 0.9% NaCl (adjusted to pH 1.5 with HCl). Propidium iodide solution (0.1 mg/µl, Sigma-Aldrich) and 50 µl of RNase (1 mg/ml) were added to the cell suspension and then incubated at 37 °C for 30 min. They were then filtered through a 48-µm mesh nylon filter and analyzed using a BD FACS Canto II at the Immunophenotyping Core Facility of the McGill University Health Centre Research Institute. Data were analyzed using FCSalyzer (Wien, Austria).
Microsatellite DNA genotyping
The FFPE blocks used for analysis were chosen based on the amount of chorionic villi (CV) they contained. Five to twelve serial 10-µm sections were cut from each block. The sections were mounted on slides and stained with hematoxylin and eosin (H&E). Under a stereomicroscope, CV were collected from the slides using Kimwipes and forceps and used for DNA extraction using the Qiagen QIAamp DNA FFPE Tissue Kit (Catalog number 56404, Hilden, Germany). Extracted DNA was quantified using a Nanodrop and loaded on a 2% agarose gel for quality evaluation and to determine the required amount for multiplex fluorescent microsatellite genotyping with the PowerPlex 16 HS System (Promega Corporation, Fitchburg, Wisconsin, USA). The reaction consisted of a short tandem repeat (STR) multiplex polymerase chain reaction (PCR) assay that amplifies DNA at 15 different STR loci and a fragment from the X and Y Amelogenin gene. DNA from the POCs and their available parents was amplified and the PCR products were resolved by capillary electrophoresis using an Applied Biosystems 3730xl DNA Analyzer (Applied Biosystems, Foster City, CA, USA) at the Centre for Applied Genomics (http://www.tcag.ca). The data were analyzed with PeakScanner, version 1.0 (Applied Biosystems, Foster City, CA, USA) and the POC alleles were compared with the parental alleles to determine their origin.
Fluorescent in situ hybridization
Fluorescent in situ hybridization (FISH) was performed on 4-μm sections that were hybridized to centromeric probes from chromosomes X, Y, and 18 as previously described [25]. On some tissues, other probes were also used. At least 100 cells for each POC were scored with each probe.
SNP microarray
Single-nucleotide polymorphism (SNP)-based whole-genome chromosomal microarray analysis was performed using the HumanCytoSNP-12 microarray (Illumina, San Diego, CA) at Invitae as previously described [26].
Statistical analysis
We estimated 95% confidence intervals (CI) using exact binomial calculations and tested for differences using Fisher’s exact test (two tailed, http://www.quantitativeskills.com/).
Results
Strategy of the analysis, main limitations of various methods, and the benefits of their combination
To determine the parental contribution to the POC, we performed comprehensive analyses using three independent methods, p57 immunohistochemistry, ploidy analysis by flow cytometry, and STR genotyping. These methods were performed systematically for all cases when appropriate materials were available. The results from the three methods, as well as those of the morphological evaluations, were compared and reconciled. Any discrepancies were resolved by repeating whichever methods led to discordant results; in some cases, discrepancies were resolved either by performing FISH on tissue sections or by performing additional simplex genotyping with appropriate markers. The systematic use of different methods along with the comparison and integration of their results allows for an accurate diagnosis and for the identification of errors obtained when relying on a single method. From our experience of genotyping ~350 FFPE molar tissues, the limitations of the various methods and the lessons we have learned can be summarized as follows.
P57KIP2 immunohistochemistry
The main limitation of this methodology stems from the quality of the tissue preparation and fixation that may lead to inappropriate p57KIP2 reactivity. Such a problem may reveal itself when the EVT and/or endometrial cells, used as an internal control, are not stained. In a subtler example, the EVT and/or endometrial cells may be less than optimally stained, and this may be accompanied by negative staining of the cytotrophoblast nuclei in a tissue that expresses p57KIP2 because in normal first-trimester trophoblastic tissues, the expression of p57KIP2 in the cytotrophoblast is much lower than that in the EVT. A more in-depth description of problems encountered with p57KIP2 immunohistochemistry and their troubleshooting are described on this excellent website (http://www.nordiqc.org).
Flow cytometry
The main problem may lie in insufficient amounts of chorionic villi in the FFPE blocks, which can prevent the detection of a triploid peak in a triploid PHM. Furthermore, tetraploid conceptions were not detected by flow cytometry under our experimental parameters because the tetraploid DNA content corresponds to the same DNA content of diploid cells in the G2 phase of the cell cycle.
STR genotyping
While this is an invaluable technique, it has numerous challenges that one needs to be aware of to benefit from this method’s full potential. The most critical problems include the following: (1) contamination with maternal tissues in POCs that have CV intermingled with maternal tissues. (2) The poor quality of the DNA extracted from FFPE tissues due to prior fixation and processing or long-term preservation. This may result in the amplification of low amounts of contaminating DNA from various sources that can in turn lead to non-maternal peaks that complicate the interpretation of the genotyping results (e.g., these peaks could be mistaken as paternal alleles in the absence of the paternal genotype). (3) The low quality of the STR genotyping and the amplification of too few markers may not allow the detection of all XX androgenetic dispermic moles. Throughout our analyses, two out of the five XX androgenetic dispermic CHM were initially misdiagnosed as monospermic CHM. After improvements to our protocol, the genotyping analysis was repeated and revealed that the two CHM were in fact androgenetic dispermic. Based on our experience, we believe that many studies underestimate the number of XX androgenetic dispermic CHM, which should theoretically account for one-third of all dispermic androgenetic CHM. Since YY conceptions do not survive early cleavage stages, the remaining androgenetic dispermic CHM (two-thirds) is expected to be XY.
Distribution of the HM genotypes and their neoplastic transformation
The analyses described above allowed us to uncover the genetic mechanisms of origin of a total of 204 sporadic moles. A summary of the genotyping results is provided in Table 1 and the results of all methods are portrayed in Supplementary Table 1. Of the 204 tissues, 114 (55.9%) were found to be androgenetic monospermic, 12 (5.9%) androgenetic dispermic, 69 (33.8%) triploid dispermic, and the remaining 9 cases (4.4%) consisted of twin or mosaic conceptions detected initially by ultrasound or p57KIP2 immunohistochemistry (Table 1).
For the analysis of the association of neoplastic transformation across HM genotypes, we only included HM that were referred to us by the RMTQ and for which complete follow-up and hCG measurements were available that allowed for accurate staging according to the FIGO guidelines [27]. We found that 48.4% (46/95) of androgenetic monospermic moles developed GTN and 1.8% (2/95) developed CC. Of the 12 androgenetic dispermic moles with complete follow-up, 91.7% (11/12) developed GTN and 25% (3/12) gave rise to CC. Among the triploid dispermic moles, 1.6% (1/62) led to a GTN, and none developed a CC. Among the 9 twin/mosaic conceptions, complete follow-up was available for 7 cases, of which 43% (3/7) developed GTN and none gave rise to a CC (Fig. 1 and Table 1). CIs for the rate of GTN do not overlap between the three genotypes, androgenetic monospermic, androgenetic dispermic, and triploid dispermic, supporting significant differences in the propensities of each group to develop GTN (Table 1). The risk of GTN is the highest for androgenetic dispermic HM, and this is significantly different from that of androgenetic monospermic HM (p value = 0.0015) and triploid dispermic HM (p value = 0). Also, the risk of GTN for androgenetic monospermic HM is higher than that of triploid dispermic HM (p value = 0). The propensity to develop CC follows a similar trend but did not reach statistical significance, given the small number of patients that developed CC (n = 5).
Among androgenetic CHM that led to GTN, we looked for an association between the severity of the GTN according to the FIGO score, low risk (≤6) vs. high risk (>6) [27], and molar genotypes, monospermic vs. dispermic. High-risk GTN was more frequent among patients with dispermic CHM, 27.2% (3/11), than among patients with monospermic CHM, 10.9% (5/46) (Table 1), suggesting an association between androgenetic dispermic CHM and high-risk GTN.
Maternal age and risk for GTN
We investigated whether maternal age affects the propensity of a HM to degenerate into a GTN. This was only possible for androgenetic monospermic moles because of the large size of this cohort. Out of our 95 patients with androgenetic monospermic CHM and complete hCG follow-up and accurate staging, 46 (48.4%) went on to develop GTN. If we only consider maternal age older than 35, 21 (65.6%) developed GTN. Out of those whose maternal age was older than 40, 14 (70.0%) developed GTN (Table 2). While CIs of the total cohort and the advanced maternal age groups do overlap, they are notably different, indicating a possible association between advanced maternal age and the propensity of an androgenetic monospermic HM to degenerate into a GTN (Table 2).
Maternal age and HM genotype
Age was available for all patients, which allowed us to include all 204 HM samples in our analysis of a possible association between maternal age and HM genotype. Out of the 114 patients who had an androgenetic monospermic HM, 6 (5.2%) were ≤20 years old at the time of HM evacuation, 45 (39.4%) were in between 21 and 30, 43 (37.7%) were in between 31 and 40, and 20 (17.5%) were older than 40 years of age. Out of the 12 patients who had an androgenetic dispermic HM, 2 (16.6%) were ≤20 years old, 6 (50%) were in between 21 and 30, 4 (33.3%) were in between 31 and 40, and none were over 40 years of age. Last, out of the 69 patients who had a triploid dispermic HM, 1 (1.6%) was ≤20 years old, 26 (37.6%) were in between 21 and 30, 42 (60.8%) were in between 31 and 40, and none were over 40 years of age (Fig. 2a, b).
We next combined our cohort of sporadic HM with another independent and well-characterized large cohort [4] consisting of a total of 297 HM, 121 CHM (106 androgenetic monospermic and 15 androgenetic dispermic), and 176 triploid dispermic PHM. In this combined cohort, we looked for an association between maternal age and HM genotype. Out of the combined cohort of patients who had an androgenetic monospermic HM, 22 (10%) were ≤20 years old, 93 (42.2%) were in between 21 and 30, 67 (30.4%) were in between 31 and 40, and 38 (17.2%) were over 40 years of age. Out of the patients who had an androgenetic dispermic HM, 2 (7.4%) were ≤20 years old, 12 (44.4%) were in between 21 and 30, 8 (29.6%) were in between 31 and 40, and 5 (18.5%) were over 40 years of age. Last, out of the patients who had a triploid dispermic HM, 24 (9.7%) were ≤20 years old, 106 (43.2%) were in between 21 and 30, 111 (45.3%) were in between 31 and 40, and 4 (1.6%) were over 40 years of age (Fig. 2c, d).
It is notable that in both cohorts (ours alone and the combined cohort), few women after the age of 40 had triploid dispermic PHM (Fig. 2). Statistical analyses of the combined cohort demonstrate that both androgenetic monospermic and dispermic CHM are significantly associated with advanced maternal age (>40) when each is compared with triploid dispermic PHM (p value = 0 and 0.00115, respectively).
History of miscarriages and HM
A recapitulation of the number of miscarriages in 106 patients with androgenetic monospermic CHM and 67 patients with triploid dispermic PHM for whom a full reproductive history was available is shown in Fig. 3. Our data show that 36.8% and 53.7% of our patients with androgenetic monospermic CHM and triploid dispermic PHM, respectively, had at least one miscarriage. This difference was not statistically significant nor was the distribution of the number of miscarriages, whether 1, 2, 3, or >3, among patients with the 2 genotypic types of HM (Fig. 3).
We next asked whether chromosomal abnormalities were at the origin of these miscarriages. To answer this question, we first reviewed the files of our patients and found that five of them had terminations of pregnancies because of fetal ultrasound abnormalities and chromosomal abnormalities identified by karyotype analysis (patients 1160, 1601, 924, 1158, and 1417) (Table 3). Then, we attempted to retrieve FFPE blocks from all available miscarriages of the 173 patients with sporadic HM (114 with androgenetic monospermic CHM and 69 with triploid dispermic PHM). We were able to retrieve 23 POCs with sufficient amounts of CV and performed SNP microarray analysis on them (Table 3 and Supplementary Fig. 1). In total (by karyotype and SNP microarray), 25 out of the 30 analyzed POCs were aneuploid [83.3%, 95% CI: 65.3–94.4%], which is higher than the frequencies of aneuploidies in women with recurrent (436/995 or 43.8%, 95% CI: 40.7–47.0%) or sporadic (3342/6491 or 51.5%, 95% CI: 50.3–52.7%) miscarriages obtained with the same type of microarray [26] and with other microarray platforms or methods [28,29,30,31,32,33,34]. This high frequency of aneuploid miscarriages remained the same (14 out of 17 POC or 82%) even after removing all cases that were referred to us as with recurrent HM and the diagnosis of some of them was revised after genotyping (underlined patient IDs in Table 3).
The ages of the patients at the time of the dilation and curettage of the molar and non-molar miscarriages are recapitulated in Table 3. These data show that the CHM occurred at an older average age (36 years) than their non-molar miscarriages (33 years), while the PHM occurred at a younger average age (32 years) than their non-molar miscarriages (34 years), which is consistent with the known increased risk for CHM with increased maternal age. Of note, 14 out of the 30 (50%) miscarriages occurred at the age of 35 or more. Furthermore, 9 out of the 13 (69%) trisomies are nonviable trisomies, known to be associated with increased maternal age [35] and most of them occurred at an age >35. Definitely, increased maternal age appears to be an important contributing factor to the aneuploid miscarriages in these patients. However, this is not the only cause because the average age of the patients at the time of non-molar miscarriages is 33–34 and the risk of any chromosomal abnormality at this age is much lower, ~1 in 156 pregnancies. Another contributing factor to the increased aneuploidies in these patients appears to be their genetic susceptibility for reproductive loss since some of these patients had few or no live births even when they were young.
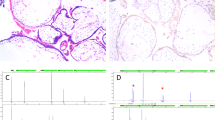
Using telomeric and pericentromeric microsatellite markers, we determined the parental and meiotic origin of the aneuploidies. In this analysis, we only investigated the origin of trisomies and triploidies because they mostly originate in the germlines or around the time of fertilization, while monosomies may also result from later anomalies during postzygotic development, and consequently, their exact origin cannot be determined. Our analysis confirmed all the trisomies revealed by SNP microarrays. In addition, it demonstrated that 9 out of the 11 (81.8%) analyzed trisomies are of maternal origin (Fig. 4), and in 2, the additional chromosomes are of paternal origin (Table 3). The latter may have originated from aneuploid male gametes or from an impaired block of polyspermy by the oocytes, leading to dispermic fertilization followed by postzygotic diploidization and loss of the other paternal chromosomes [36]. Of the six triploidies identified among the 30 POCs, 4 were found to be digynic miscarriages (Fig. 5) and 2 were found dispermic PHM (Table 3). Notably, both anomalies are due to oocyte defects.
The miscarriages in (a, b, c, e, f, i) are due to failure of MI. The miscarriages in (d, g, h) are due to failure of MII. All markers except D16S678 and D16S753 are pericentromeric. The x-axis represents size in basepairs and the y axis represents peak height. Both have been omitted for clarity. POC stands for product of conception.
The miscarriage in (a) is due to failure of MII and the miscarriage in (c) is due to failure of MI. The markers shown for the miscarriages in (b) and (d) demonstrate the maternal origin of the triploidies. Other markers from the pericentromeric regions were performed on the POCs analyzed in (b) and (d) and demonstrated that they resulted from the failure of MI and MII, respectively (data not shown). The x axis represents size in basepairs and the y axis represents peak height. Both have been omitted for clarity. POC stands for product of conception.
Altogether, our data suggest that patients with complete or partial HM and miscarriages have a higher frequency of aneuploid miscarriages than women with one or more miscarriages, and most of these aneuploidies are of maternal meiotic origin.
Discussion
In this study, we used several approaches to comprehensively characterize the genotypes of 204 HM from patients, mostly referred to us by the RMTQ, and 30 of their non-molar miscarriages. We revisited risk factors for HM and GTN across the different HM genotypes and investigated the genetic causes of their non-molar miscarriages.
In our analysis, we found a higher frequency of GTN (53.3%) as compared with other studies from western countries where 14–28% of patients with CHM are reported to develop GTN [37,38,39]. This difference is clearly due to a referral bias and the fact that patients’ enrolment in the RMTQ is made on a voluntary basis by healthcare professionals. Consequently, this may have favored enrolling severe cases from physicians seeking help or a second opinion in the management of their patients and therefore increased the risk of GTN in our patients with CHM.
It is well known that androgenetic, both monospermic and dispermic, CHM is more prone to GTN than triploid dispermic PHM [37,38,39], and this is replicated in our analysis, in which 53.3% of CHM and 1.6% of PHM lead to GTN. However, among androgenetic CHM, reports about the differences in the propensity of monospermic vs. dispermic genotypes to neoplasia have been less consistent [40,41,42,43,44,45,46,47]. While many studies found higher frequencies of GTN among dispermic vs. monospermic androgenetic CHM, most did not reach statistical significance because of the small number of patients with androgenetic dispermic CHM [40,41,42,43,44]. To date, only Baasanjav et al. [46] reached a significant increase in GTN after dispermic as compared with monospermic CHM in their own samples but not in a meta-analysis after combining all previously described cases. In an attempt to answer this debated question, we evaluated the incidence of GTN across the various genotypes of HM. Our data show that androgenetic dispermic moles have a higher risk for GTN (91.7%) than androgenetic monospermic moles (48.4%) and therefore confirm previous findings [46]. We also demonstrate that GTN after androgenetic dispermic CHM has higher FIGO risk score (score > 6) (33 vs. 6%) and is at higher risk for CC (25 vs. 1.8%) than GTN after androgenetic monospermic CHM. Furthermore, our data demonstrate that frequency of GTN in patients with androgenetic monospermic moles increases with increased maternal age, and this finding is in agreement with previous reports [48,49,50,51].
Increased maternal age is a well-known risk factor significantly associated with CHM [48, 52, 53]. In studies where the genotypes of the moles were determined, this association was reported with androgenetic CHM [4]. This was also confirmed in our cohort of 204 HM and after combining our cases with 297 HM samples reported by Banet et al. [4]. In addition, analyzing the combined cohort revealed an association between increased maternal age and androgenetic dispermic CHM, which was not seen in our 12 patients with androgenetic dispermic cases and has not been previously reported.
Aside from maternal age, the second highest risk factor for HM that was demonstrated in several studies and populations is a history of miscarriages [17,18,19]. In one of these studies [19], miscarriages were found associated with both histological types of HM. However, the cause of non-molar miscarriages in women with sporadic HM is unknown. From our cohort of patients with sporadic HM, 36.8% of those with androgenetic monospermic CHM and 53.7% of those with triploid dispermic PHM had at least one miscarriage. The rate of miscarriages among our patients is higher than that previously reported and is due, in our judgment, to the following facts: (i) some patients were recruited from the recurrent miscarriage clinic; ii) others were referred to us with two HM, one of which was found to be a non-molar miscarriage after genotyping; (iii) our follow-up on the reproductive history of some of our patients continued for several years after the diagnosis of their sporadic HM. Upon analyzing the miscarriages of our patients, 83.3% were found aneuploid, which is higher than the frequency of aneuploidy in women with recurrent (43.8%) or sporadic (53.7%) miscarriages [26, 28,29,30,31,32,33,34]. We next determined the parental origin of trisomies and triploidies and demonstrated that 9 out of 13 (69%) of the trisomies and 4 out of 6 (67%) of the triploidies resulted from failure of female meiosis I or II. Representative results are illustrated in Figs. 4, 5. These data suggest that a genetic susceptibility for defects in meiosis I and II may underlie the etiology of HM and aneuploid miscarriages in these patients. Our findings are in agreement with the fact that increased maternal age (>35) is the most important risk factor for both HM (CHM and PHM) [48, 53, 54] and aneuploid miscarriages [35, 55]. Indeed, age-specific rates of HM and miscarriages follow similar J-shaped curves with a slight increase in teenagers and a steep increase after the age of 35 [56,57,58,59,60]. Furthermore, in a recent study documenting the identification of three novel meiotic genes underlying the etiology of recurrent androgenetic monospermic moles, the patients and their female siblings also had miscarriages, which further support the relationship between meiotic defects, androgenetic CHM, and miscarriages in some patients [61].
Change history
01 February 2020
An amendment to this paper has been published and can be accessed via a link at the top of the paper.
12 February 2020
A Correction to this paper has been published: https://doi.org/10.1038/s41379-020-0487-2
References
Szulman AE, Surti U. The syndromes of hydatidiform mole. II. Morphologic evolution of the complete and partial mole. Am J Obstet Gynecol. 1978;132:20–7.
Vassilakos P, Kajii T. Letter: Hydatidiform mole: two entities. Lancet. 1976;1:259.
Kovacs BW, Shahbahrami B, Tast DE, Curtin JP. Molecular genetic analysis of complete hydatidiform moles. Cancer Genet Cytogenet. 1991;54:143–52.
Banet N, DeScipio C, Murphy KM, Beierl K, Adams E, Vang R, et al. Characteristics of hydatidiform moles: analysis of a prospective series with p57 immunohistochemistry and molecular genotyping. Mod Pathol. 2014;27:238–54.
Lipata F, Parkash V, Talmor M, Bell S, Chen S, Maric V, et al. Precise DNA genotyping diagnosis of hydatidiform mole. Obstet Gynecol. 2010;115:784–94.
Vang R, Gupta M, Wu LS, Yemelyanova AV, Kurman RJ, Murphy KM, et al. Diagnostic reproducibility of hydatidiform moles: ancillary techniques (p57 immunohistochemistry and molecular genotyping) improve morphologic diagnosis. Am J Surg Pathol. 2012;36:443–53.
Colgan TJ, Chang MC, Nanji S, Kolomietz E. DNA genotyping of suspected partial hydatidiform moles detects clinically significant aneuploidy. Int J Gynecol Pathol. 2017;36:217–21.
Furtado LV, Paxton CN, Jama MA, Tripp SR, Wilson AR, Lyon E, et al. Diagnostic utility of microsatellite genotyping for molar pregnancy testing. Arch Pathol Lab Med. 2013;137:55–63.
Nguyen NM, Zhang L, Reddy R, Dery C, Arseneau J, Cheung A, et al. Comprehensive genotype-phenotype correlations between NLRP7 mutations and the balance between embryonic tissue differentiation and trophoblastic proliferation. J Med Genet. 2014;51:623–34.
Brown L, Mount S, Reddy R, Slim R, Wong C, Jobanputra V, et al. Recurrent pregnancy loss in a woman with NLRP7 mutation: not all molar pregnancies can be easily classified as either "partial" or "complete" hydatidiform moles. Int J Gynecol Pathol. 2013;32:399–405.
Sebire NJ, Savage PM, Seckl MJ, Fisher RA. Histopathological features of biparental complete hydatidiform moles in women with NLRP7 mutations. Placenta. 2013;34:50–6.
Hayward BE, De Vos M, Talati N, Abdollahi MR, Taylor GR, Meyer E, et al. Genetic and epigenetic analysis of recurrent hydatidiform mole. Hum Mutat. 2009;30:E629–39.
Judson H, Hayward BE, Sheridan E, Bonthron DT. A global disorder of imprinting in the human female germ line. Nature. 2002;416:539–42.
Reddy R, Akoury E, Phuong Nguyen NM, Abdul-Rahman OA, Dery C, Gupta N, et al. Report of four new patients with protein-truncating mutations in C6orf221/KHDC3L and colocalization with NLRP7. Eur J Hum Genet. 2013;21:957–64.
Parry DA, Logan CV, Hayward BE, Shires M, Landolsi H, Diggle C, et al. Mutations causing familial biparental hydatidiform mole implicate c6orf221 as a possible regulator of genomic imprinting in the human oocyte. Am J Hum Genet. 2011;89:451–8.
Qian J, Nguyen NMP, Rezaei M, Huang B, Tao Y, Zhang X, et al. Biallelic PADI6 variants linking infertility, miscarriages, and hydatidiform moles. Eur J Hum Genet. 2018;26:1007–13.
Messerli ML, Lilienfeld AM, Parmley T, Woodruff JD, Rosenshein NB. Risk factors for gestational trophoblastic neoplasia. Am J Obstet Gynecol. 1985;153:294–300.
Parazzini F, La Vecchia C, Pampallona S, Franceschi S. Reproductive patterns and the risk of gestational trophoblastic disease. Am J Obstet Gynecol. 1985;152:866–70.
Parazzini F, Mangili G, La Vecchia C, Negri E, Bocciolone L, Fasoli M. Risk factors for gestational trophoblastic disease: a separate analysis of complete and partial hydatidiform moles. Obstet Gynecol. 1991;78:1039–45.
Sauthier P, Breguet M, Rozenholc A, Sauthier M. Quebec Trophoblastic Disease Registry: how to make an easy-to-use dynamic database. Int J Gynecol Cancer. 2015;25:729–33.
Ngan HY, Bender H, Benedet JL, Jones H, Montruccoli GC, Pecorelli S, et al. Gestational trophoblastic neoplasia, FIGO 2000 staging and classification. Int J Gynaecol Obstet. 2003;83(Suppl 1):175–7.
Castrillon DH, Sun D, Weremowicz S, Fisher RA, Crum CP, Genest DR. Discrimination of complete hydatidiform mole from its mimics by immunohistochemistry of the paternally imprinted gene product p57KIP2. Am J Surg Pathol. 2001;25:1225–30.
Hedley DW. Flow cytometry using paraffin-embedded tissue: five years on. Cytometry. 1989;10:229–41.
Khawajkie Y, Buckett W, Nguyen NMP, Mechtouf N, Ao A, Arseneau J, et al. Recurrent triploid digynic conceptions and mature ovarian teratomas: Are they different manifestations of the same genetic defect? Genes Chromosomes Cancer. 2017;56:832–40.
Surti U, Hoffner L, Kolthoff M, Dunn J, Hunt J, Sniezek L, et al. Persistent gestational trophoblastic disease after an androgenetic/biparental fetal chimera: a case report and review. Int J Gynecol Pathol. 2006;25:366–72.
Sahoo T, Dzidic N, Strecker MN, Commander S, Travis MK, Doherty C, et al. Comprehensive genetic analysis of pregnancy loss by chromosomal microarrays: outcomes, benefits, and challenges. Genet Med. 2017;19:83–9.
Ngan HY, Seckl MJ, Berkowitz RS, Xiang Y, Golfier F, Sekharan PK, et al. Update on the diagnosis and management of gestational trophoblastic disease. Int J Gynaecol Obstet. 2015;131(Suppl 2):S123–6.
Maslow BS, Budinetz T, Sueldo C, Anspach E, Engmann L, Benadiva C, et al. Single-nucleotide polymorphism-microarray ploidy analysis of paraffin-embedded products of conception in recurrent pregnancy loss evaluations. Obstet Gynecol. 2015;126:175–81.
Hassold T, Chen N, Funkhouser J, Jooss T, Manuel B, Matsuura J, et al. A cytogenetic study of 1000 spontaneous abortions. Ann Hum Genet. 1980;44:151–78.
Eiben B, Bartels I, Bahr-Porsch S, Borgmann S, Gatz G, Gellert G, et al. Cytogenetic analysis of 750 spontaneous abortions with the direct-preparation method of chorionic villi and its implications for studying genetic causes of pregnancy wastage. Am J Hum Genet. 1990;47:656–63.
Stephenson MD, Awartani KA, Robinson WP. Cytogenetic analysis of miscarriages from couples with recurrent miscarriage: a case-control study. Hum Reprod. 2002;17:446–51.
Shearer BM, Thorland EC, Carlson AW, Jalal SM, Ketterling RP. Reflex fluorescent in situ hybridization testing for unsuccessful product of conception cultures: a retrospective analysis of 5555 samples attempted by conventional cytogenetics and fluorescent in situ hybridization. Genet Med. 2011;13:545–52.
Menten B, Swerts K, Delle Chiaie B, Janssens S, Buysse K, Philippe J, et al. Array comparative genomic hybridization and flow cytometry analysis of spontaneous abortions and mors in utero samples. BMC Med Genet. 2009;10:89.
Robberecht C, Schuddinck V, Fryns JP, Vermeesch JR. Diagnosis of miscarriages by molecular karyotyping: benefits and pitfalls. Genet Med. 2009;11:646–54.
Grande M, Borrell A, Garcia-Posada R, Borobio V, Munoz M, Creus M, et al. The effect of maternal age on chromosomal anomaly rate and spectrum in recurrent miscarriage. Hum Reprod. 2012;27:3109–17.
Golubovsky MD. Postzygotic diploidization of triploids as a source of unusual cases of mosaicism, chimerism and twinning. Hum Reprod. 2003;18:236–42.
Garner EI, Goldstein DP, Feltmate CM, Berkowitz RS. Gestational trophoblastic disease. Clin Obstet Gynecol. 2007;50:112–22.
Golfier F, Raudrant D, Frappart L, Mathian B, Guastalla JP, Trillet-Lenoir V, et al. First epidemiological data from the French Trophoblastic Disease Reference Center. Am J Obstet Gynecol. 2007;196:172 e1–5.
Sebire NJ, Lindsay I. Current issues in the histopathology of gestational trophoblastic tumors. Fetal Pediatr Pathol. 2010;29:30–44.
Wake N, Seki T, Fujita H, Okubo H, Sakai K, Okuyama K, et al. Malignant potential of homozygous and heterozygous complete moles. Cancer Res. 1984;44:1226–30.
Wake N, Fujino T, Hoshi S, Shinkai N, Sakai K, Kato H, et al. The propensity to malignancy of dispermic heterozygous moles. Placenta. 1987;8:319–26.
Lawler SD, Fisher RA. Genetic studies in hydatidiform mole with clinical correlations. Placenta. 1987;8:77–88.
Lawler SD, Fisher RA, Dent J. A prospective genetic study of complete and partial hydatidiform moles. Am J Obstet Gynecol. 1991;164:1270–7.
Cho S, Kim SJ. Genetic study of hydatidiform moles by restriction fragment length polymorphisms (RFLPs) analysis. J Korean Med Sci. 1993;8:446–52.
Cheung AN, Sit AS, Chung LP, Ngan HY, O'Hanlan K, Wong LC, et al. Detection of heterozygous XY complete hydatidiform mole by chromosome in situ hybridization. Gynecol Oncol. 1994;55:386–92.
Baasanjav B, Usui H, Kihara M, Kaku H, Nakada E, Tate S, et al. The risk of post-molar gestational trophoblastic neoplasia is higher in heterozygous than in homozygous complete hydatidiform moles. Hum Reprod. 2010;25:1183–91.
Kaneki E, Kobayashi H, Hirakawa T, Matsuda T, Kato H, Wake N. Incidence of postmolar gestational trophoblastic disease in androgenetic moles and the morphological features associated with low risk postmolar gestational trophoblastic disease. Cancer Sci. 2010;101:1717–21.
Savage PM, Sita-Lumsden A, Dickson S, Iyer R, Everard J, Coleman R, et al. The relationship of maternal age to molar pregnancy incidence, risks for chemotherapy and subsequent pregnancy outcome. J Obstet Gynaecol. 2013;33:406–11.
Xia ZF, Song HZ, Tang MY. Risk of malignancy and prognosis using a provisional scoring system in hydatidiform mole. Chin Med J (Engl). 1980;93:605–12.
Tow WS. The influence of the primary treatment of hydatidiform mole on its subsequent course. J Obstet Gynaecol Br Common. 1966;73:544–52.
Tsukamoto N, Iwasaka T, Kashimura Y, Uchino H, Kashimura M, Matsuyama T. Gestational trophoblastic disease in women aged 50 or more. Gynecol Oncol. 1985;20:53–61.
Graham IH, Fajardo AM, Richards RL. Epidemiological study of complete and partial hydatidiform mole in Abu Dhabi: influence age and ethnic group. J Clin Pathol. 1990;43:661–4.
Sebire NJ, Foskett M, Fisher RA, Rees H, Seckl M, Newlands E. Risk of partial and complete hydatidiform molar pregnancy in relation to maternal age. BJOG. 2002;109:99–102.
Yen S, MacMahon B. Epidemiologic features of trophoblastic disease. Am J Obstet Gynecol. 1968;101:126–32.
Choi TY, Lee HM, Park WK, Jeong SY, Moon HS. Spontaneous abortion and recurrent miscarriage: a comparison of cytogenetic diagnosis in 250 cases. Obstet Gynecol Sci. 2014;57:518–25.
Magnus MC, Wilcox AJ, Morken NH, Weinberg CR, Haberg SE. Role of maternal age and pregnancy history in risk of miscarriage: prospective register based study. Br Med J. 2019;364:l869.
Matalon M, Modan B. Epidemiologic aspects of hydatidiform mole in Israel. Am J Obstet Gynecol. 1972;112:107–12.
Grimes DA. Epidemiology of gestational trophoblastic disease. Am J Obstet Gynecol. 1984;150:309–18.
George L, Granath F, Johansson AL, Olander B, Cnattingius S. Risks of repeated miscarriage. Paediatr Perinat Epidemiol. 2006;20:119–26.
Nybo Andersen AM, Wohlfahrt J, Christens P, Olsen J, Melbye M. Maternal age and fetal loss: population based register linkage study. Br Med J. 2000;320:1708–12.
Nguyen NMP, Ge ZJ, Reddy R, Fahiminiya S, Sauthier P, Bagga R, et al. Causative mutations and mechanism of androgenetic hydatidiform moles. Am J Hum Genet. 2018;103:740–51.
Acknowledgements
The authors would like to thank Dr. Olga Basso for her help with the statistical analysis. This study was supported by the Canadian Institutes of Health Research to R.S. (MOP-130364, MOP 102469, and MOP 86546). We also express our gratitude to the Pathology Departments of the following health centers for providing archived products of conception for analyses: McGill University Health Centre, Centre Hospitalier de l’Université de Montréal, Centre Hospitalier Universitaire de Sainte-Justine, Centre de la Santé de Laval, Hôpital Pierre Le Gardeur, Centre Hospitalier Régional de Rimouski, Centre de Santé et de Services Sociaux de Trois Rivières, London Health Science Centre, Hôpital Pierre Boucher, Hôpital Maisonneuve Rosemont, Hôpital Sacré-Coeur, Hôpital Anna-Laberge, Hôpital Général du Lakeshore, Jewish General Hospital, Hôpital Honoré Mercier, Credit Valley Hospital, Hôpital Saint Jean sur Richelieu, Hôpital Regional Saint Jerome, Hôpital Sainte-Mary, and Windsor Regional Hospital.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Khawajkie, Y., Mechtouf, N., Nguyen, N.M.P. et al. Comprehensive analysis of 204 sporadic hydatidiform moles: revisiting risk factors and their correlations with the molar genotypes. Mod Pathol 33, 880–892 (2020). https://doi.org/10.1038/s41379-019-0432-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41379-019-0432-4
This article is cited by
-
Aneuploidy is frequent in heterozygous diploid and triploid hydatidiform moles
Scientific Reports (2024)
-
Recurrent Androgenetic Complete Hydatidiform Moles with p57KIP2-Positive in a Chinese Family
Reproductive Sciences (2022)
-
Genetic screening of Chinese patients with hydatidiform mole by whole-exome sequencing and comprehensive analysis
Journal of Assisted Reproduction and Genetics (2022)
-
The genetics of recurrent hydatidiform moles in Mexico: further evidence of a strong founder effect for one mutation in NLRP7 and its widespread
Journal of Assisted Reproduction and Genetics (2021)
-
Parental contribution to trisomy in heterozygous androgenetic complete moles
Scientific Reports (2020)