Abstract
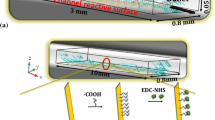
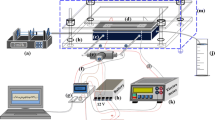
In heterogeneous microfluidic immunosensors, enhanced capture efficiencies of the antigens (Ag) in the carrier fluids by the surface-immobilized antibodies (Ab) facilitate lower detection limits and thus early detection of disease. Capture efficiency depends on the interplay of transport, reaction parameters and the geometry of the system. A detailed analysis on the enhanced capture efficiencies due to secondary flows in heterogeneous immunosensors has not received significant attention and is the theme of the present work. We conducted a systematic study to observe the significance of secondary forces on the capture efficiency, manifested as the average surface concentration (Cs,avg), in serpentine channels of different lengths (l) and radius of curvature (Rc) as a function of the Reynolds number (Re). Experimental observations were validated with numerical simulations. Micro-PIV studies at different planes and sections of the serpentine microchannels were conducted and matched with the simulated velocity profiles. Further investigation of the process and the geometrical parameters was conducted using numerical simulation and the behaviour of Cs,avg as a function of Re and Rc was plotted for different cases. A highlight of the present work are correlations of Cs,avg as a function of the Dean number (De), as well as its constituents (Re and α). The scientific studies of the geometrical and process parameters which affect the analyte capture advance the understanding of the phenomena and the proposed engineering correlations would be useful in the design of more efficient flow-based heterogeneous immunosensors.











Similar content being viewed by others
References
Art MS, Noblitt SD, Krummel AT et al (2018) IR-compatible PDMS microfluidic devices for monitoring of enzyme kinetics. Anal Chimica Acta 1021:95–102
Bange A, Halsall B, Heineman WR (2005) Microfluidic immunosensor systems. Biosens Bioelectron 20:2488–2503
Bhuvana M, Narayanan JS, Dharuman V et al (2013) Gold surface supported spherical liposome–gold nano-particle nano-composite for label free DNA sensing. Biosens Bioelectron 41:802–808
Brown AP, Anson FC (1977) Cyclic and differential pulse voltammetric behavior of reactants confined to the electrode surface. Anal Chem 49:1589–1595
Chauhan R, Singh J, Solanki PR (2016) Label-free piezoelectric immunosensor decorated with gold nanoparticles: kinetic analysis and biosensing application. Sens Actu B 222:804–814
Chen S et al (2019) microfluidic device directly fabricated on screen-printed electrodes for ultrasensitive electrochemical sensing of PSA. Nano Res Let 14:71
Chepyala R, Panda S (2013) Tunable surface free energies of functionalized molecular layers on Si surfaces for microfluidic immunosensor applications. App Surf Sci 271:77–85
Chepyala R, Panda S (2014) Zeta potential and Reynolds number correlations for electrolytic solutions in microfluidic immunosensor. Micro Nano 18:1329–1339
Di CM, Ting YY, Yang DZ et al (2019) Microchannel with stacked microbeads for separation o Plasma from whole blood. Chinese J Anal Chem 47(5):661–668
Dravid AN, Smith KA, Merrill EW, Brian PLT (1971) Effect of secondary fluid motion on laminar flow heat transfer in helically coiled tubes. AlChE J 17:1114–1122
Foley JO, Hossein AM, Fu E et al (2008) Experimental and model investigation of the time-dependent 2-dimensional distribution of binding in a herringbone microchannel. Lab Chip 8:557–564
Fujii T (2002) PDMS-based microfluidic devices for biomedical applications. Micro Electron Eng 61–62:907–914
Golden JP, Floyd-Smith TM, Rott DR et al (2007) Target delivery in a microfluidic immunosensor. Biosens Bioelectron 22:2763–2767
Hessel V, Lowe H, Schonfeld F (2005) Micromixers—a review on passive and active mixing principles. Chem Engg Sci 60:2479–2501
Hu G, Gao Y, Li D (2007) Modeling micropatterned antigen-antibody binding kinetics in a microfluidic chip. Biosens Bioelectron 22:1403–1409
Ibii T, Kaieda M, Hatakeyama S et al (2010) Direct immobilization of gold-binding antibody fragments for immunosensor applications. Anal Chem 82:4229–4235
Imran H, Manikandan PN, Prabhu D et al (2019a) Ultra selective label free electrochemical detection of cancer prognostic p53-antibody at DNA functionalized grapheme. Sens Bio-sens Res 23:100261
Imran H, Manikandan PN, Dharuman V (2019b) Ultra-sensitive and selective label free electrochemical DNA detection at layer-by-layer self-assembled graphene oxide and vesicle liposome nano-architecture. J Electro Anal Chem 835:10–21
Jeon W, Shin BC (2009) Design and simulation of passive mixing in microfluidic systems with geometric variations. Chem Engg J 152:575–582
Kadilak AL, Ying L, Shrestha S et al (2014) Selective deposition of chemically- bonded gold electrodes onto PDMS microchannel side walls. J Electroanal Chem 727:141–147
Kalita P, Singh J, Singh MK et al (2012) Ring like self-assembled Ni nanoparticles based biosensor for food toxin detection. Appl Phy Let 100:093702
Kirtland JD, McGraw GJ, Stroock AD (2006) Mass transfer to reactive boundaries from steady three-dimensional flows in microchannels. Phys Fluids 18:073602-1-13
Klink MJ, Iwuoha EI, Ebenso EE (2011) The electro-catalytic and redox-mediator effects of nanostructured PDMA-PSA modified-electrodes as phenol derivative sensors. Int J Electrochem Sci 6:2429–2442
Kumar V, Paraschivoiu M, Nigam KDP (2011) Single-phase fluid flow and mixing in microchannels. Chem Eng Sci 66:1329–1373
Lebedev K, Mafe S, Stroeve P (2006) Convection, diffusion and reaction in a surface-based biosensor: modeling of cooperativity and binding site competition on the surface and in the hydrogel. J Colloid Int Sci 296:527–537
Li N, Schwartz M, Zanetti CI (2009) PDMS compound adsorption in context. J Biomol Screen 14(2):194–202
Lynn NS, Homola JJ (2015) Biosensor enhancement using grooved micromixers: Part I, numerical studies. Anal Chem 87:5516–5523
Lynn NS, Sipova H, Adam P, Homola JJ (2013) Enhancement of affinity-based biosensors: effect of sensing chamber geometry on sensitivity. Lab Chip 13:1413–1421
Lynn NS, Lopez JIM, Bockova M et al (2014) Biosensing enhancement using passive mixing structures for microarray-based sensors. Biosens Bioelectron 54:506–514
Lynn NS, Bockova JM, Adam P, Homola J (2015) Biosensor enhancement using grooved micromixers: Part II, experimental studies. Anal Chem 87:5524–5530
Mandal MM, Kumar V, Nigam KDP (2010) Augmentation of heat transfer performance in coiled flow inverter vis-a-vis conventional heat exchanger. Chem Eng Sci 65:999–1007
Mandal MM, Aggarwal P, Nigam KDP (2011) Liquid-liquid mixing in coiled flow inverter. Ind Eng Chem Res 50:13230–13235
Meinhart CD, Wereley ST, Santiago JG (1999) PIV measurements of a microchannel flow. Exp Fluids 27:414–419
Meng-Di C et al (2019) Microchannel with stacked microbeads for separation of plasma from whole blood. Chin J Anal Chem 47(5):661–668
Nelson KE, Foley JO, Yager P (2007) Concentration gradient immunoassay. 1. An immunoassay based on interdiffusion and surface binding in a microchannel. Anal Chem 79:3542–3548
Rath D, Panda S (2015a) Enhanced capture efficiencies of antigens in immunosensors. Chem Eng J 60:657–670
Rath D, Panda S (2015b) Contribution of rotational diffusivity towards the transport of antigens in heterogeneous immunosensors. Analyst 140:6579–6587
Rath D, Panda S (2016) Correlation of capture efficiency with the geometry, transport, and reaction parameters in heterogeneous immunosensors. Langmuir 32:1410–1418
Rath D, Kumar S, Panda S (2012) Enhancement of antigen–antibody kinetics on nanotextured silicon surfaces in mixed non-flow systems. Mat Sci Eng C 32:2223–2229
Reverberi R, Reverberi L (2007) Factors affecting the antigen-antibody reaction. Blood Transfus 5:227–240
Singh J, Kalita P, Singh MK et al (2011) Nanostructured nickel oxide-chitosan film for application to cholesterol sensor. Appl Phy Let 98:123702
Solanki PR, Kaushik A, Ansari AA et al (2008) Zinc oxide-chitosan nanobiocomposite for urea sensor. Appl Phys Let 93:163903
Squires TM, Messinger RJ, Manalis SR (2008) Making it stick: convection, reaction and diffusion in surface-based biosensors. Nat Biotechnol 26:417–426
Srivastava S et al (2011) A self assembled monolayer based microfluidic sensor for ures detection. Nanoscale 3:2971–2977
Vijayendran RA, Leckband DE (2001) A quantitative qssessment of heterogeneity for surface-immobilized proteins. Anal Chem 73:471–480
Vijayendran RA, Motsegood KM, Beebe DJ, Leckband DE (2003) Evaluation of a three-dimensional micromixer in a surface-based biosensor. Langmuir 19:1824–1828
Wang S, Liu K, Liu J (2011) Highly efficient capture of circulating tumor cells by using nanostructured silicon substrates with integrated chaotic micromixers. Angew Chem Int Ed 50:3084–3088
Zhang F et al (2015) A microfluidic love-wave biosensing device for PSA detection based on an Aptamer Beacon Probe. Sensors 15:13839–13850
Zhang D, Ma J, Meng X et al (2019) Electrochemical aptamer-based microsensor for real-time monitoring of adenosine in vivo. Anal Chim Acta 1076:55–63
Zheng X, Silber ZH (2008) Measurement of velocity profiles in a rectangular microchannel with aspect ratio α = 0.35. Exp Fluids 44:951–959
Acknowledgements
The authors acknowledge financial support from the Ministry of Electronics and Information Technology, Government of India (Grant Number 2(4)/2014-PEGD (IPIIW)). The use of the micro-PIV unit (supported by the FIST program of the Department of Science and Technology, Government of India) in the PG Research Laboratory in the Department of Chemical Engineering is acknowledged. The help of Dr. Satyendra Kumar with the processing of the antibody and antigen samples is acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Verma, S., Panda, S. Correlations of the capture efficiency with the Dean number and its constituents in heterogeneous microfluidic immunosensors. Microfluid Nanofluid 24, 9 (2020). https://doi.org/10.1007/s10404-019-2312-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10404-019-2312-0