Abstract
Data regarding the lower diagnostic threshold for gastric graft-versus-host disease is lacking. The aim of this study was to review a cohort of gastric biopsies taken to evaluate for graft-versus-host disease, and to correlate histologic findings with clinical and endoscopic evidence of graft-versus-host disease as well as biopsy findings from other locations to define a lower diagnostic threshold for gastric graft-versus-host disease. Gastric biopsies were evaluated for the maximum number of apoptotic bodies per 10 contiguous gastric pits, presence of ≥1 apoptotic body per biopsy (NIH criteria), and presence of gastric pit dropout and/or ulceration. To evaluate histologic specificity, sixty gastric biopsies from non-stem cell transplant patients were selected as a control group. Clinical information was collected from chart review. The study group consisted of 65 gastric biopsies from 52 stem cell transplant patients. The mean apoptotic count per 10 contiguous gastric pits for stem cell transplant biopsies was 1.8 (range 0–8) and for control cases 1.0 (range 0–5). Nineteen stem cell transplant biopsies (29%) had ≥1 apoptotic body per biopsy and only a single case had >6 apoptotic bodies per 10 contiguous gastric pits. When the NIH guidelines were combined with presence of at least two apoptotic bodies per 10 contiguous gastric pits, this cutoff point was significantly associated with treatment for graft-versus-host disease (OR = 9.4, 95% CI = 1.7–176.7, p = 0.04) and evidence of extraintestinal graft-versus-host disease (OR = 3.2, 95% CI = 1.1–10.7, p = 0.04). The diagnostic specificity for our proposed cutoff value is 94%. We present criteria for the lower diagnostic threshold of gastric graft-versus-host disease, which uses a lower apoptotic cutoff value than has been utilized in colonic biopsies. Although sensitivity remains a challenge for gastric graft-versus-host disease biopsies, this newly proposed cutoff provides higher specificity than NIH guidelines alone and better correlates with clinical evidence of graft-versus-host disease.
Similar content being viewed by others
Intoduction
Graft-versus-host disease is a complication of hematopoietic stem cell transplantation associated with significant morbidity and mortality. Graft-versus-host disease commonly involves the skin, liver, and gastrointestinal tract, and appropriate diagnosis is critical as patients require increased immunosuppressive therapy for resolution of symptoms [1].
Diagnosis of graft-versus-host disease relies on a combination of clinical, endoscopic, and histologic features. Characteristic histologic features in the gastrointestinal tract include crypt/gland apoptosis, crypt/gland dropout, and ulceration. The 1974 Lerner system has been commonly used to histologically grade gastrointestinal graft-versus-host disease and requires the presence of only a single apoptotic body to establish the diagnosis [2]. However, apoptosis in the gastrointestinal tract is nonspecific and can also be seen in association with infection, conditioning chemotherapy regimens, and certain medications [3,4,5,6,7,8]. Furthermore, overdiagnosis of graft-versus-host disease may lead to unnecessary escalation of immunosuppressive therapy, putting patients at increased risk of opportunistic infection [1].
Because of the low specificity of the Lerner system, recent studies have reexamined the lower histologic threshold for diagnosis of graft-versus-host disease. Some authors have proposed using >6 apoptotic bodies per 10 contiguous crypts to establish a diagnosis of graft-versus-host disease, with cases having ≤6 apoptotic bodies per 10 contiguous crypts considered as indeterminate for graft-versus-host disease [9, 10]. The 2014 National Institute of Health (NIH) consensus guidelines, on the other hand, recommend the presence of ≥1 apoptotic body per biopsy fragment to establish the diagnosis of graft-versus-host disease [11]. The criteria for these grading systems are largely based on colonic biopsies and some studies have shown that the histologic features of graft-versus-host disease tend to be less severe in the stomach compared with the lower gastrointestinal tract [12]. Therefore, data for the lower diagnostic threshold of graft-versus-host disease in gastric biopsies is still largely lacking [11].
The aim of this study was to retrospectively review a cohort of gastric biopsies taken to evaluate for graft-versus-host disease in stem cell transplant patients, and to correlate gastric histologic findings with clinical and endoscopic evidence of graft-versus-host disease, as well as biopsy findings from other locations to define a lower diagnostic threshold for gastric graft-versus-host disease.
Material and methods
Case selection and clinical information
Approval of this study was obtained from the Institutional Review Board. The surgical pathology database at our institution was retrospectively searched for gastric biopsies taken to evaluate for graft-versus-host disease from 2008 to 2013. Biopsies from asymptomatic patients and solid organ transplant patients were excluded from the study.
Corresponding clinical information, including age, gender, underlying disease, conditioning regimen, type of the transplant, time elapsed since the transplant, symptoms at the time of biopsy, endoscopic findings, evidence of infection, evidence of extraintestinal graft-versus-host disease, medication history, and treatment for graft-versus-host disease was collected from chart review. Treatment for graft-versus-host disease following endoscopy was defined as either initiation of a new immunosuppressant agent or an increase in the dose of immunosuppressant agents that the patient was receiving prior to biopsy. Response to treatment was defined as resolution of symptoms within 1 month of treatment initiation. A skin or liver biopsy diagnostic of graft-versus-host disease or characteristic clinical features of graft-versus-host disease in the absence of biopsy were considered as evidence of extraintestinal graft-versus-host disease.
Histologic evaluation
All biopsies were reviewed blindly by one gastrointestinal pathologist (CEH). Gastric biopsies were evaluated for the maximum number of apoptotic bodies per 10 contiguous gastric pits, presence of ≥1 apoptotic body per biopsy fragment, and presence of gastric pit dropout and/or ulceration. The number of biopsy fragments per specimen and number of serial sections examined were documented. Corresponding biopsies from the small bowel or colon were also reviewed and regraded using the modified Lerner system [9]: Indeterminate for graft-versus-host disease = ≤6 apoptotic bodies per 10 contiguous crypts with no evidence of crypt loss, grade 1 = >6 apoptotic bodies per 10 contiguous crypts with no evidence of crypt loss, grade 2 = apoptosis with individual crypt loss, grade 3 = apoptosis with contiguous crypt loss, and grade 4 = extensive crypt loss with ulceration. Corresponding esophageal biopsies were assessed for the presence or absence of apoptosis. Previously performed cytomegalovirus immunohistochemical stains and Warthin–Starry stains for Helicobacter pylori were reviewed when available. Original diagnoses were later collected from corresponding pathology reports.
Selection of control cases
Ten biopsy cases each of normal gastric mucosa, H. pylori gastritis, chronic inactive H. pylori negative gastritis, gastric intestinal metaplasia, reactive gastropathy, and iron pill gastritis from non-stem cell transplant patients were selected as a control group. Similar to the study group, biopsies from the control group were evaluated for the maximum number of apoptotic bodies per 10 contiguous gastric pits, presence of ≥1 apoptotic body per biopsy fragment, and presence of gastric pit dropout and/or ulceration.
Statistical methods
T test or one-way ANOVA was used to compare continuous variables between groups and chi-square test or Fisher’s exact test was used to compare categorical variables by use of the Prism statistical program (GraphPad, 2015, San Diego, CA, USA). Univariate logistic regression analysis was performed using Excel (Microsoft, 2010, Redmond, WA, USA).
Results
Demographic, clinical, and endoscopic information
The study group consisted of 65 gastric biopsies from 52 stem cell transplant patients. Six patients had two sets of biopsies included in the study, two patients had three sets of biopsies, and one had four sets of biopsies. Thirty-one patients were male and 21 were female (M:F 1.5:1), with mean age at the time of biopsy of 51.7 years (range 20–68 years). The underlying diagnoses for which stem cell transplant was performed included: acute myelogenous leukemia (n = 16), multiple myeloma (n = 12), non-Hodgkin lymphoma (n = 9), acute lymphocytic leukemia (n = 5), myelodysplastic syndrome (n = 5), aplastic anemia (n = 2), chronic myelogenous leukemia (n = 1), myeloproliferative disorder (n = 1), and myelofibrosis (n = 1). Forty-five patients underwent allogenic stem cell transplant, while seven patients underwent autologous stem cell transplant. The source of the transplanted stem cells was bone marrow in seven cases, peripheral blood in 42 cases, and cord blood in one case; in two patients, clinical information regarding the source of the transplanted stem cells was not available. Time elapsed since the transplant to biopsy ranged from 15 to 3650 days (mean = 430 days).
All patients were symptomatic at the time of the biopsy, with several patients complaining of more than one symptom. Symptoms included: nausea (n = 36), diarrhea/loose stools (n = 34), vomiting (n = 23), weight loss (n = 9), abdominal/epigastric pain (n = 6), dysphagia/odynophagia (n = 5), anorexia/loss of appetite (n = 5), heartburn (n = 2), melena (n = 2), dry oral mucosa (n = 2), fever (n = 1), and muscle aches (n = 1). Reports were available for 49 endoscopy procedures. Endoscopic findings in the stomach in order of frequency were: mucosal erythema (n = 26), normal (n = 16), ulceration (n = 7), nodularity (n = 4), edema (n = 2), mucosal flattening (n = 2), bile reflux (n = 2), and bleeding (n = 1). Some patients showed presence of more than one endoscopic mucosal abnormality.
Histology of stem cell transplant patient biopsies
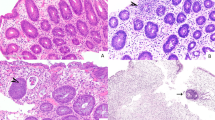
The mean number of gastric biopsy fragments per specimen was 4.1 (range = 1–9) and the mean number of serial sections examined was 9.0 (range = 6–12). The mean apoptotic count per 10 contiguous gastric pits for all biopsies was 1.8 (range 0–8). Nineteen biopsies (29%) had ≥1 apoptotic body per biopsy fragment and only a single case had >6 apoptotic bodies per 10 contiguous gastric pits. Only five cases (8%) had evidence of gastric pit dropout and no cases with apoptosis showed evidence of mucosal ulceration (Fig. 1). All cases with gastric pit dropout had ≥1 apoptotic body per biopsy fragment and the mean apoptotic count per 10 contiguous gastric pits for these cases was 4 (range 1–8). All patients with gastric pit dropout had evidence of graft-versus-host disease at extraintestinal sites and three of the cases had evidence of graft-versus-host disease in corresponding colon and/or small bowel biopsies.
This case was reclassified as indeterminate for graft versus host disease (a). Gastric biopsy from stem cell transplant patient with dilated withered pits containing eosinophilic luminal debris (arrows) consistent with gastric pit dropout (b). Control case showing a single gastric pit with luminal debris (arrow). The pit lacks the withered appearance noted in b (c). Control case with intestinal metaplasia showing gastric pit apoptosis (arrow) (d).
Cytomegalovirus immunohistochemical stain was performed on 34 biopsies and was negative in all cases. No histologic features indicative of viral infection were observed in the hematoxylin and eosin stained slides for the remaining 31 gastric biopsies. Warthin–Starry stain was performed on all 65 biopsies and was positive for H. pylori organisms in two cases. Additional histologic findings in the gastric biopsies included: mild chronic inflammation (n = 16), intestinal metaplasia (n = 6), active chronic gastritis (n = 2), focal neutrophilic inflammation (n = 2), erosion (n = 1), and reactive gastropathy (n = 1).
The original graft-versus-host disease grades rendered on gastric biopsies were: one case grade 3, 14 cases grade 1, one case indeterminate for graft-versus-host disease, and 48 cases negative for graft-versus-host disease. There was a single case for which the original pathologist did not give a graft-versus-host disease grade at the time of diagnosis.
Sixty-three cases had biopsies from the gastrointestinal tract at sites other than the stomach and of these nongastric biopsies, 27 (43%) showed no evidence of graft-versus-host disease. The maximum graft-versus-host disease grade for the remaining biopsies from nongastric sites were: indeterminate for graft-versus-host disease (n = 2), grade 1 (n = 24), grade 2 (n = 2), grade 3 (n = 5), and grade 4 (n = 3).
Treatment, presence of confounding factors, and presence of extraintestinal graft-versus-host disease
Treatment was initiated after the biopsy in 45 cases (69%), while in 18 cases there was no change in the immunosuppressant therapy. No corresponding treatment information was available for two patients. Of the 45 treated cases, 40 patients (89%) responded to treatment, four patients (9%) showed no response, and one patient had no clinical record available for treatment response.
At the time of biopsy, ten patients had clinical evidence of infection: H. pylori (n = 2, diagnosed via biopsy as described above, 2 and 0 apoptotic bodies per 10 contiguous gastric pits), C. difficile (n = 2, 2 and 3 apoptotic bodies per 10 contiguous gastric pits), cytomegalovirus (n = 2, 1 apoptotic body per 10 contiguous gastric pits each), human herpesvirus 6 (n = 1, 3 apoptotic bodies per 10 contiguous gastric pits), herpes simplex virus (n = 1, 0 apoptotic bodies per 10 contiguous gastric pits), cytomegalovirus/herpes simplex virus (n = 1, 1 apoptotic body per 10 contiguous gastric pits), and Shigelloides (n = 1, 2 apoptotic bodies per 10 contiguous gastric pits). Diagnosis of cytomegalovirus infection was made clinically via serology.
Twenty patients were on mycophenolate at the time of biopsy, and 50 patients were on proton pump inhibitor. Four patients had biopsy performed <21 days post transplant; two underwent allogeneic peripheral blood stem cell transplant and two autologous peripheral blood stem cell transplant.
Thirty patients (46%) had evidence of extraintestinal graft-versus-host disease involving, skin (n = 29), liver (n = 8), lung (n = 2), oral cavity (n = 5), eye (n = 3), and pancreas (n = 1). Several patients had more than one extraintestinal site involved by graft-versus-host disease.
Comparison of clinical and histologic features in patients treated for graft-versus-host disease versus untreated patients
Compared with patients who were untreated, patients treated for graft-versus-host disease were significantly more likely to have evidence of graft-versus-host disease in corresponding gastrointestinal tract biopsies taken from nongastric locations (80 vs. 0%, p < 0.0001), as well as evidence of graft-versus-host disease at extraintesinal sites (60 vs. 17%, p = 0.002). Patients treated for graft-versus-host disease also had a significantly higher maximum apoptotic counts per 10 contiguous gastric pits compared with untreated patients (2.1 vs. 0.9, p = 0.01). Full histologic and clinical data for treated versus untreated patients is shown in Table 1.
Comparison of histologic cutoffs points in correlation with clinical and histologic evidence of graft-versus-host disease
To determine a suitable threshold for diagnosis of graft-versus-host disease in the stomach, different cutoff points were examined in correlation with clinical evidence of graft-versus-host disease via logistic regression, including evidence of graft-versus-host disease in nongastric gastrointestinal biopsies, evidence of extraintestinal graft-versus-host disease, and treatment for graft-versus-host disease. For this analysis, cases with gastric pit dropout were considered positive for graft-versus-host disease regardless of the degree of apoptosis. Presence of ≥1 apoptotic body per biopsy fragment (NIH criteria) by itself was not significantly associated with any of the selected criteria. An apoptotic cutoff value of ≥2 apoptotic bodies per 10 contiguous gastric pits was associated with decision to treat but was not associated with other surrogate markers of graft-versus-host disease. When the NIH guidelines were combined with presence of at least 2 apoptotic bodies per 10 contiguous gastric pits, this cutoff point was significantly associated with treatment for graft-versus-host disease (OR = 9.4, 95% CI = 1.7–176.7, p = 0.04) and evidence of extraintestinal graft-versus-host disease (OR = 3.2, 95% CI = 1.1–10.7, p = 0.04) (Table 2).
Histology of control cases and calculation of sensitivity, specificity, and accuracy
The majority of control cases (67%) showed at least one apoptotic body. The mean apoptotic count per 10 contiguous gastric pits for all control cases was 1.0 (range 0–5) and six cases (10%) had ≥1 apoptotic body per biopsy fragment. No control cases showed evidence of pit dropout, although two cases did have a single gastric pit with luminal apoptotic debris. However, the cells lining these pits lacked the attenuation seen in cases of pit dropout in graft-versus-host disease (Fig. 1c). Four cases (7%) had at least 2 apoptotic bodies per 10 contiguous gastric pits and ≥1 apoptotic body per biopsy fragment. Control cases with intestinal metaplasia showed the highest apoptotic count per 10 contiguous gastric pits, but there was no significant difference in the apoptotic counts between control groups (p = 0.79) (Fig. 1d). Compared with biopsies from stem cell transplant patients, the control group had similar apoptotic counts to patients who were not treated for graft-versus-host disease (p = 0.75) and significantly lower apoptotic counts compared with patients who were treated for graft-versus-host disease (p = 0.0002). Full histologic data for the control groups is shown in Table 3.
For calculation of sensitivity, specificity, and accuracy, stem cell transplant patients treated for graft-versus-host disease were considered positive cases, and control cases and untreated stem cell transplant patients were considered as negative. The presence of any apoptosis had a sensitivity of 82%, specificity of 36%, and accuracy of 53%. The NIH criteria had a sensitivity of 36%, specificity of 90%, and accuracy of 70%. The presence of at least 2 apoptotic bodies per 10 contiguous gastric pits and ≥1 apoptotic body per biopsy had a sensitivity of 33%, specificity of 94%, and accuracy of 72%.
Reclassification of biopsies according to new criteria
Following identification of the optimal lower threshold for diagnosis of gastric graft-versus-host disease, gastric biopsies were regraded according to our newly proposed criteria: negative for graft-versus-host disease = no gastric pit apoptosis; Indeterminate for graft-versus-host disease ≤1 apoptotic body per biopsy fragment or ≥1 apoptotic body per biopsy fragment without at least 2 apoptotic bodies per 10 contiguous gastric pits and no gastric pit dropout; Positive for graft-versus-host disease = at least 1 apoptotic body per biopsy fragment and at least 2 apoptotic bodies per 10 contiguous gastric pits or presence of gastric pit dropout with any apoptosis.
Seventeen cases were reclassified as negative for graft-versus-host disease; fifteen of these cases had originally been called negative for graft-versus-host disease and two cases had been called grade 1 graft-versus-host disease but showed no gastric pit apoptosis on retrospective review. Thirty cases were reclassified as indeterminate for graft-versus-host disease; twenty-three of these cases were originally called negative for graft-versus-host disease, one was called indeterminate for graft-versus-host disease, and six were called grade 1 graft-versus-host disease. Eighteen cases were reclassified as positive for graft-versus-host disease; ten had originally been called negative for graft-versus-host disease, including three cases with gastric pit dropout and the remaining eight had originally been called positive for graft-versus-host disease (grades 1–3). Of the ten cases originally called negative for graft-versus-host disease and reclassified as positive for graft-versus-host disease, seven had corresponding nongastric biopsies that were called positive for graft-versus-host disease, two had evidence of extraintestinal graft-versus-host disease, and one received treatment prior to biopsy with no further escalation of immunosuppression following biopsy. Among the cases reclassified as positive for graft-versus-host disease, cases originally called negative for graft-versus-host disease had significantly lower apoptotic counts per 10 contiguous gastric pits compared to cases that were originally called positive for graft-versus-host disease (2.8 vs. 4.5, p = 0.025).
Cases reclassified as positive for graft-versus-host disease were significantly more likely to have received treatment for graft-versus-host disease but response to treatment was similar among groups (Table 4). Eight patients reclassified as negative for graft-versus-host disease were still treated with increased immunosuppressive therapy; five of these patients had evidence of graft-versus-host disease in corresponding nongastric biopsies, one was treated due to noncompliance with medications, one was treated because of evidence of lung graft-versus-host disease, and one patient was treated because the biopsy had originally been called positive for graft-versus-host disease.
Of the nine patients with indeterminate for graft-versus-host disease biopsies that were not treated for graft-versus-host disease, clinical diagnoses included reflux in two patients, mycophenolate toxicity, cytomegalovirus/herpes simplex virus infection, upper respiratory infection, and irritable bowel syndrome. Two patients’ clinical diagnoses were unknown as symptoms spontaneously resolved without treatment and one additional patient had received immunosuppressive therapy prior to biopsy for suspected graft-versus-host disease but received no additional escalation of immunosuppressive therapy following biopsy. Three patients had indeterminate for graft-versus-host disease histology on gastric biopsies and no evidence of graft-versus-host disease in corresponding nongastric biopsies or at extraintestinal sites. All three patients were treated with increased immunosuppression and responded to therapy. Two patients with indeterminate for graft-versus-host disease biopsies who received immunosuppressive therapy were refractory to treatment. One patient had continued gastrointestinal symptoms and was transferred to hospice care. The other patient continued to have melena secondary to gastric and duodenal erosions, which resolved 1.5 months after initiation of immunosuppression.
One patient with a biopsy positive for graft-versus-host disease was not treated. The patient had received treatment for graft-versus-host disease 2.5 months prior to the biopsy. There was no further escalation in immunosuppressive therapy and the patient’s symptoms resolved. This was the only patient reclassified as positive for graft-versus-host disease who did not have evidence of graft-versus-host disease in nongastric biopsies or at extraintestinal sites. Two patients with biopsies positive for graft-versus-host disease were refractory to immunosuppressive therapy. One patient was transferred to hospice care and the other patient’s symptoms improved with addition of tincture of opium 6 months after biopsy.
Discussion
Epithelial apoptosis, glandular dropout, and mucosal ulceration are well established as the histologic hallmarks of gastrointestinal graft-versus-host disease. However, given the low specificity of glandular apoptosis in the gastrointestinal tract, the histologic threshold for diagnosis of gastrointestinal graft-versus-host disease remains debated. Some recent studies have addressed this issue. Lin et al. proposed using >6 apoptotic bodies per 10 contiguous crypts to establish a diagnosis of graft-versus-host disease, and subsequent studies have further supported this threshold [9, 10, 13]. The 2014 NIH guidelines recommend the presence of ≥1 apoptotic body per biopsy fragment to establish a diagnosis [11]. These studies and consequent recommendations have all largely been based on colonic biopsies.
To define suitable diagnostic criteria for gastric graft-versus-host disease, we retrospectively reviewed 65 gastric biopsies from 52 stem cell transplant patients for histologic features of graft-versus-host disease including: maximum number of apoptotic bodies per 10 contiguous gastric pits, presence of ≥1 apoptotic body per biopsy fragment, and presence of gastric pit dropout or ulceration and compared with a control group of 60 gastric biopsies from non-stem cell transplant patients. The mean apoptotic count per 10 contiguous gastric pits for all gastric biopsies from stem cell transplant patients was 1.8 and only one case showed >6 apoptotic bodies per 10 contiguous gastric pits. The mean apoptotic count per 10 contiguous gastric pits for the control cases was 1.0, and patients treated for graft-versus-host disease had significantly higher apoptotic bodies per 10 contiguous gastric pits compared with the control cases. A minority of cases (8%) in our cohort showed gastric pit dropout, but this feature was specific for graft-versus-host disease. Prior studies have shown that the prevalence and histologic grade of graft-versus-host disease tends to be lower in upper gastrointestinal tract biopsies compared with lower gastrointestinal tract biopsies [12, 14, 15]. Our findings further confirm that the histologic features of graft-versus-host disease in the stomach tend to be subtle consisting primarily of rare gastric pit apoptosis.
Because of the lower apoptotic counts within the gastric biopsies, cutoff values lower than 6 apoptotic bodies per 10 contiguous gastric pits were assessed. An apoptotic cutoff value of ≥2 apoptotic bodies per 10 contiguous gastric pits was associated with decision to treat but was not associated with other surrogate markers of graft-versus-host disease such as evidence of graft-versus-host disease in nongastric gastrointestinal biopsies and extraintestinal graft-versus-host disease. The 2014 NIH guidelines (≥1 apoptotic body per biopsy fragment) did not show significant association with any surrogate markers of graft-versus-host disease. However, when a combination of NIH guidelines and presence of ≥2 apoptotic bodies per 10 contiguous gastric pits were used, statistically significant correlation was reached with decision to treat and presence of graft-versus-host disease at other gastrointestinal sites. This newly proposed cutoff provides higher specificity and accuracy than NIH guidelines alone and therefore better correlates with clinical evidence of graft-versus-host disease.
We recently examined the significance of using the indeterminate for graft-versus-host disease classification in colonic biopsies and found patients classified as indeterminate for graft-versus-host disease were more likely to be managed conservatively versus those classified as positive for graft-versus-host disease (75 vs. 100% treatment) [10]. Based on reclassification of gastric biopsies we found similar rates of treatment in the as indeterminate for graft-versus-host disease group versus cases positive for graft-versus-host disease (70 vs. 94% treatment). Nine patients in our cohort with as indeterminate for graft-versus-host disease histology on gastric biopsy symptomatically resolved without increased immunosuppression. In addition, the majority of our control cases (67%) had at least one apoptotic body. These findings again reinforce that not every biopsy with glandular apoptosis is diagnostic of graft-versus-host disease and use of indeterminate for graft-versus-host disease terminology can prevent unnecessary escalation of immunosuppressive therapy in the appropriate clinical context.
Interestingly over half (56%) of our cases that we reclassified as positive for graft-versus-host disease were originally called negative for graft-versus-host disease, including three cases with gastric pit dropout. The majority of these cases (90%) had corresponding nongastric biopsies that were called positive for graft-versus-host disease or evidence of extraintestinal graft-versus-host disease. In addition, the calculated sensitivity of our newly proposed histologic cutoff value is only 33%. These findings demonstrate that the diagnostic sensitivity of gastric biopsies for graft-versus-host disease is low and standardized criteria for diagnosis of gastric graft-versus-host disease are lacking. As such, anatomic sites other than the stomach should preferentially be targeted for biopsy to establish the diagnosis of graft-versus-host disease. However, in rare instances gastric biopsies may provide diagnostic utility. Three patients in our cohort had evidence of indeterminate for graft-versus-host disease histology on gastric biopsies with no evidence graft-versus-host disease in nongastric biopsies or at extraintestinal sites. All three patients required increased immunosuppression and symptomatically responded to therapy, suggesting that these cases truly represented graft-versus-host disease. Therefore, establishing guidelines for the diagnosis of gastric graft-versus-host disease is still valuable and pathologists should familiarize themselves with the histologic features of gastric graft-versus-host disease to avoid underdiagnosis.
Washington et al. [16] published one of the few papers specifically evaluating the histologic features and diagnostic criteria for gastric graft-versus-host disease. In the study, a blind review of 56 gastric biopsies showed apoptosis, gland destruction, sparse inflammatory infiltrate, and granular eosinophilic debris are the most useful features in establishing a diagnosis of gastric graft-versus-host disease. The study also reported stomach involvement to be more severe than colonic involvement; however the authors acknowledge this finding may be due to a selection bias. While many of our patients complained of upper gastrointestinal symptoms, our study was not designed to only include patients with upper gastrointestinal symptoms and therefore may explain our disparate findings. In addition, while Washington et al. did show moderate interobserver agreement in the diagnosis of gastric graft-versus-host disease, this study did not define a minimum threshold to establish the diagnosis of gastric graft-versus-host disease. To best of our knowledge our study is the first to evaluate the threshold for diagnosis of graft-versus-host disease based primarily on gastric biopsy material.
Given the retrospective design of our study, it was limited by the lack of the standardization of the biopsy material in terms of location within the gastrointestinal tract and number of the biopsies taken. However, there was no significant difference in number of biopsies examined in patients that were treated for graft-versus-host disease versus those that were untreated. Likewise, we were not able to control for presence of confounding factors such as evidence of infection or certain medications, but again there was no significant difference in presence of these confounding factors in patients that were treated versus those that were untreated.
For the first time we present criteria for the lower diagnostic threshold of gastric graft-versus-host disease. While the stomach is not the optimal site to obtain biopsies for diagnosis of graft-versus-host disease, occasionally gastric biopsies may provide the only histologic evidence for a diagnosis of graft-versus-host disease. Pathologists should familiarize themselves with the subtle histologic features of gastric graft-versus-host disease to avoid underdiagnosis. Prospective studies utilizing gastric biopsy materials to evaluate the histologic features and diagnostic cutoffs for gastric graft-versus-host disease are necessary to confirm the findings of this study.
References
Arnaout K, Patel N, Jain M, El-Amm J, Amro F, Tabbara IA. Complications of allogeneic hematopoietic stem cell transplantation. Cancer Investig 2014;32:349–62.
Lerner KG, Kao GF, Storb R, Buckner CD, Clift RA, Thomas ED. Histopathology of graft-vs.-host reaction (GvHR) in human recipients of marrow from HL-A-matched sibling donors. Transpl Proc. 1974;6:367–71.
Selbst MK, Ahrens WA, Robert ME, Friedman A, Proctor DD, Jain D. Spectrum of histologic changes in colonic biopsies in patients treated with mycophenolate mofetil. Mod Pathol. 2009;22:737–43.
Brito GAC, Fujji J, Carneiro-Filho BA, Lima AAM, Obrig T, Guerrant RL. Mechanism of Clostridium difficile toxin A-induced apoptosis in T84 cells. J Infect Dis. 2002;186:1438–47.
McCole DF, Eckmann L, Laurent F, Kagnoff MF. Intestinal epithelial cell apoptosis following Cryptosporidium parvum infection. Infect Immun. 2000;68:1710–3.
Snover DC. Mucosal damage simulating acute graft-versus-host reaction in cytomegalovirus colitis. Transplantation. 1985;39:669–70.
Epstein RJ, McDonald GB, Sale GE, Shulman HM, Thomas ED. The diagnostic accuracy of the rectal biopsy in acute graft-versus-host disease: a prospective study of thirteen patients. Gastroenterology. 1980;78:764–71.
Welch DC, Wirth PS, Goldenring JR, Ness E, Jagasia M, Washington K. Gastric graft-versus-host disease revisited: does proton pump inhibitor therapy affect endoscopic gastric biopsy interpretation? Am J Surg Pathol. 2006;30:444–9.
Lin J, Fan R, Zhao Z, Cummings OW, Chen S. Is the presence of 6 or fewer crypt apoptotic bodies sufficient for diagnosis of graft versus host disease? A decade of experience at a single institution. Am J Surg Pathol. 2013;37:539–47.
Rowan DJ, Hartley CP, Carrillo-Polanco LF, Oshima K, Hagen CE. Diagnostic phrasing is independently correlated with the decision to treat for graft-versus-host disease: retrospective review of colon biopsies with rare apoptosis. Histopathology. 2016;69:802–11.
Shulman HM, Cardona DM, Greenson JK, Hingorani S, Horn T, Huber E, et al. NIH Consensus development project on criteria for clinical trials in chronic graft-versus-host disease: II. The 2014 Pathology Working Group Report. Biol Blood Marrow Transpl. 2015;21:589–603.
Ma C, Maluf HM, Liu T-C. Acute graft-versus-host disease is more prevalent and severe in the lower than the upper gastrointestinal tract. Hum Pathol. 2015;46:1480–7.
Gomez AJ, Arai S, Higgins JP, Kambham N. Clinicopathologic threshold of acute colorectal graft-versus-host disease. Arch Pathol Lab Med. 2016;140:570–7.
Ross WA, Ghosh S, Dekovich AA, Liu S, Ayers GD, Cleary KR, et al. Endoscopic biopsy diagnosis of acute gastrointestinal graft-versus-host disease: rectosigmoid biopsies are more sensitive than upper gastrointestinal biopsies. Am J Gastroenterol. 2008;103:982–9.
Nydegger A, Catto-Smith AG, Tiedemann K, Hardikar W. Diagnosis of gastrointestinal graft-versus-host disease-is rectal biopsy enough? Pediatr Blood Cancer. 2007;48:561–6.
Washington K, Bentley RC, Green A, Olson J, Treem WR, Krigman HR. Gastric graft-versus-host disease: a blinded histologic study. Am J Surg Pathol. 1997;21:1037–46.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mostafa, M., Hartley, C.P. & Hagen, C.E. Evaluation of the lower histologic threshold for gastric graft versus host disease. Mod Pathol 33, 962–970 (2020). https://doi.org/10.1038/s41379-019-0421-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41379-019-0421-7