Abstract
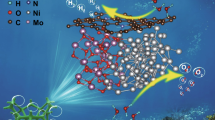
Pd catalysts supported on carbon have interesting features for chlorophenols-bearing water treatment by hydrodechlorination, including high stability, activity, and tunable support. In this work, a set of materials based on reduced graphene oxide (rGO) with different degrees of reduction, surface oxygen groups, nitrogen doping, and specific surface area were decorated with Pd nanoparticles, with diameter average size between 5 and 50 nm. Ethanol, hydrazine monohydrate, and sodium borohydride were used as reducing agents, and Pd(AcO)2 was used as a Pd precursor to obtain Pd/rGO nanocomposites in our developed one-pot synthesis approach. A thorough characterization revealed that a similar degree of reduction was obtained for sodium borohydride at 25 °C and hydrazine at 60 °C, although hydrazine also led to nitrogen doping. Pd nanoparticles were homogenously nucleated on the surface of the GO and the obtained nanocomposites were tested as catalysts for hydrodechlorination of 4-chlorophenol in the aqueous phase. The materials with a higher degree of reduction showed the best specific catalytic activity at 70 °C. However, the turnover frequency (TOF) only increased with the reduction degree for the N-doped Pd/rGO, indicating the important role of the nitrogenous functionalities in the catalytic activity. Therefore, the N doping of Pd/rGO catalysts is a feasible strategy to synthesize catalysts highly active for hydrodechlorination.

Graphical abstract







Similar content being viewed by others
References
Alonso-Morales N, Ruiz-Garcia C, Palomar J, Heras F, Calvo L, Rodriguez JJ, Gilarranz MA (2017) Hollow nitrogen- or boron-doped carbon submicrospheres with a porous shell: preparation and application as supports for hydrodechlorination catalysts. Ind Eng Chem Res 56:7665–7674
Arrigo R, Schuster ME, Xie ZL, Yi YM, Wowsnick G, Sun LL, Hermann KE, Friedrich M, Kast P, Havecker M, Knop-Gericke A, Schlogl R (2015) Nature of the N-Pd interaction in nitrogen-doped carbon nanotube catalysts. ACS Catal 5:2740–2753
Baeza JA, Calvo L, Gilarranz MA, Mohedano AF, Casas JA, Rodriguez JJ (2012) Catalytic behavior of size-controlled palladium nanoparticles in the hydrodechlorination of 4-chlorophenol in aqueous phase. J Catal 293:85–93
Baeza JA, Alonso-Morales N, Calvo L, Heras F, Rodriguez JJ, Gilarranz MA (2015) Hydrodechlorination activity of catalysts based on nitrogen-doped carbons from low-density polyethylene. Carbon 87:444–452
Bokobza L, Bruneel J-L, Couzi M (2014) Raman spectroscopy as a tool for the analysis of carbon-based materials (highly oriented pyrolitic graphite, multilayer graphene and multiwall carbon nanotubes) and of some of their elastomeric composites. Vib Spectrosc 74:57–63
Calvo L, Gilarranz MA, Casas JA, Mohedano AF, Rodríguez JJ (2009) Hydrodechlorination of 4-chlorophenol in water with formic acid using a Pd/activated carbon catalyst. J Hazard Mater 161:842–847
Chiou J-R, Lai B-H, Hsu K-C, Chen D-H (2013) One-pot green synthesis of silver/iron oxide composite nanoparticles for 4-nitrophenol reduction. J Hazard Mater 248–249:394–400
Cruz-Silva R, Morelos-Gomez A, Kim H-I, Jang H-K, Tristan F, Vega-Diaz S, Rajukumar LP, Elias AL, Perea-Lopez N, Suhr J, Endo M, Terrones M (2014) Super-stretchable graphene oxide macroscopic fibers with outstanding knotability fabricated by dry film scrolling. ACS Nano 8:5959–5967
Deng H, Fan G, Wang C, Zhang L (2014) Aqueous phase catalytic hydrodechlorination of 4-chlorophenol over palladium deposited on reduced graphene oxide. Catal Commun 46:219–223
Diaz E, Casas JA, Mohedano AF, Calvo L, Gilarranz MA, Rodriguez JJ (2009) Kinetics of 4-chlorophenol hydrodechlorination with alumina and activated carbon-supported Pd and Rh catalysts. Ind Eng Chem Res 48:3351–3358
Dreyer DR, Park S, Bielawski CW, Ruoff RS (2010) The chemistry of graphene oxide. Chem Soc Rev 39:228–240
Gao X, Jang J, Nagase S (2010) Hydrazine and thermal reduction of graphene oxide: reaction mechanisms, product structures, and reaction design. J Phys Chem C 114:832–842
Gomez-Sainero LM, Seoane XL, Fierro JLG, Arcoya A (2002) Liquid-phase hydrodechlorination of CCI4 to CHCl3 on Pd/carbon catalysts: Nature and role of Pd active species. J Catal 209:279–288
Green AA, Hersam MC (2009) Solution phase production of graphene with controlled thickness via density differentiation. Nano Lett 9:4031–4036
Grzyb B, Gryglewicz S, Sliwak A, Diez N, Machnikowski J, Gryglewicz G (2016) Guanidine, amitrole and imidazole as nitrogen dopants for the synthesis of N-graphenes. RSC Adv 6:15782–15787
Guo S, Dong S (2011) Graphene nanosheet: synthesis, molecular engineering, thin film, hybrids, and energy and analytical applications. Chem Soc Rev 40:2644–2672
Ham H., Tran Van K., Park N.-H., So D.S., Lee J.-W., Na H.G., Kwon Y.J., Cho H.Y., Kim H.W. (2014) Freeze-drying-induced changes in the properties of graphene oxides, Nanotechnology, 25:4830-4840.
Hu B, Ding K, Wu T, Zhou X, Fan H, Jiang T, Wang Q, Han B (2010) Shape controlled synthesis of palladium nanocrystals by combination of oleylamine and alkylammonium alkylcarbamate and their catalytic activity. Chem.Comm. 46:8552–8554
Jiang H (2011) Chemical preparation of graphene-based nanomaterials and their applications in chemical and biological sensors. Small 7:2413–2427
Kim SK, Kim C, Lee JH, Kim J, Lee H, Moon SH (2013) Performance of shape-controlled Pd nanoparticles in the selective hydrogenation of acetylene. J Catal 306:146–154
Krishnankutty N, Vannice MA (1995) The effect of pretreatment on Pd/C catalysts. I. Adsorption and absorption properties. J. Catal. 155:312–326
Lan L, Du F, Xia C (2016) The reaction mechanism for highly effective hydrodechlorination of p-chlorophenol over a Pd/CNTs catalyst. RSC Adv 6:109023–109029
Long D, Li W, Ling L, Miyawaki J, Mochida I, Yoon SH (2010) Preparation of nitrogen-doped graphene sheets by a combined chemical and hydrothermal reduction of graphene oxide. Langmuir 26:16096–16102
López GP, Castner DG, Ratner BD (1991) XPS O 1s binding energies for polymers containing hydroxyl, ether, ketone and ester groups. Surf Interface Anal 17:267–272
Mamtani K, Jain D, Co AC, Ozkan US (2017) Investigation of chloride poisoning resistance for nitrogen-doped carbon nanostructures as oxygen depolarized cathode catalysts in acidic media. Catal Lett 147:2903–2909
Marcano DC, Kosynkin DV, Berlin JM, Sinitskii A, Sun Z, Slesarev A, Alemany LB, Lu W, Tour JM (2010) Improved synthesis of graphene oxide. ACS Nano 4:4806–4814
Navalon S, Dhakshinamoorthy A, Alvaro M, Garcia H (2016) Metal nanoparticles supported on two-dimensional graphenes as heterogeneous catalysts. Coord Chem Rev 312:99–148
Novoselov KS, Geim AK, Morozov SV, Jiang D, Zhang Y, Dubonos SV, Grigorieva IV, Firsov AA (2004) Electric field effect in atomically thin carbon films. Science 306:666–669
Park S, Ruoff RS (2009) Chemical methods for the production of graphenes. Nat Nanotechnol 4:217–224
Park S, An J, Potts JR (2011) A. Ve200lamakanni, S. Murali, R.S. Ruoff, Hydrazine-reduction of graphite- and graphene oxide. Carbon 49:3019–3023
Pei S, Cheng H-M (2012) The reduction of graphene oxide. Carbon 50:3210–3228
Peng-Gang R, Ding-Xiang Y, Xu J, Tao C, Zhong-Ming L (2011) Temperature dependence of graphene oxide reduced by hydrazine hydrate. Nanotechnology 22:055705
Pietrzak R (2009) XPS study and physico-chemical properties of nitrogen-enriched microporous activated carbon from high volatile bituminous coal. Fuel 88:1871–1877
Rahul R, Singh RK, Bera B, Devivaraprasad R, Neergat M (2015) The role of surface oxygenated-species and adsorbed hydrogen in the oxygen reduction reaction (ORR) mechanism and product selectivity on Pd-based catalysts in acid media. Phys Chem Chem Phys 17:15146–15155
Riedl C, Coletti C, Starke U (2010) Structural and electronic properties of epitaxial graphene on SiC(0 001): a review of growth, characterization, transfer doping and hydrogen intercalation. J Phys D Appl Phys 43:374009
Ruan G, Sun Z, Peng Z, Tour JM (2011) Growth of graphene from food, insects, and waste. ACS Nano 5:7601–7607
Ruditskiy A, Choi SI, Peng HC, Xia YN (2014) Shape-controlled metal nanocrystals for catalytic applications. MRS Bull 39:727–737
Ruiz-Garcia C, Perez-Carvajal J, Berenguer-Murcia A, Darder M, Aranda P, Cazorla-Amoros D, Ruiz-Hitzky E (2013) Clay-supported graphene materials: application to hydrogen storage. Phys Chem Chem Phys 15:18635–18641
Ruiz-Garcia C, Darder M, Aranda P, Ruiz-Hitzky E (2014) Toward a green way for the chemical production of supported graphenes using porous solids. J Mater Chem A 2:2009–2017
Ruiz-García C, Heras F, Calvo L, Alonso-Morales N, Rodriguez JJ, Gilarranz MA (2018a) Platinum and N-doped carbon nanostructures as catalysts in hydrodechlorination reactions. Appl Catal B Environ 238:609–617
Ruiz-Garcia C, Heras F, Alonso-Morales N, Calvo L, Rodriguez JJ, Gilarranz MA (2018b) Enhancement of the activity of Pd/C catalysts in aqueous phase hydrodechlorination through doping of carbon supports. Catal Sci Technol 8:2598–2605
Ruiz-García C, Heras F, Gilarranz MA, Aranda P, Ruiz-Hitzky E (2018c) Sepiolite-carbon nanocomposites doped with Pd as improving catalysts for hydrodechlorination processes. Appl Clay Sci 161:132–138
Ruiz-García C, Heras F, Calvo L, Alonso-Morales N, Rodriguez JJ, Gilarranz MA (2019) N-doped CMK-3 carbons supporting palladium nanoparticles as catalysts for hydrodechlorination. Ind Eng Chem Res 58:4355–4363
She X, Yang Q, Yao F, Zhong Y, Ren W, Chen F, Sue J, Ma Y, Fe Z, Wang D (2019) Electrocatalytic hydrodechlorination of 4-chlorophenol on Pd supported multi-walled carbon nanotubes particle electrodes. Chem Eng J 358:903–911
Sing KSW, Rouquerol F, Rouquerol J, Llewellyn P (2014) Adsorption by powders and porous solids (Second Edition). Academic Press, Oxford
Stobinski L, Lesiak B, Malolepszy A, Mazurkiewicz M, Mierzwa B, Zemek J, Jiricek P, Bieloshapka I (2014) Graphene oxide and reduced graphene oxide studied by the XRD, TEM and electron spectroscopy methods. J Electron Spectros Relat Phenomena 195:145–154
Terrones M (2009) Nanotubes unzipped. Nature 458:845–846
Terrones M, Botello-Mendez AR, Campos-Delgado J, Lopez-Urias F, Vega-Cantu YI, Rodriguez-Macias FJ, Elias AL, Munoz-Sandoval E, Cano-Marquez AG, Charlier J-C, Terrones H (2010) Graphene and graphite nanoribbons: morphology, properties, synthesis, defects and applications. Nano Today 5:351–372
Vu THT, Tran TTT, Le HNT, Tran LT, Nguyen PHT, Nguyen MD, Quynh BN (2016) Synthesis of Pt/rGO catalysts with two different reducing agents and their methanol electrooxidation activity. Mater Res Bull 73:197–203
Wagner C.D., Riggs W.M., Davis L.E., Moulder J.F. (1979) Handbook of x-ray photoelectron spectroscopy, Perkin-Elmer
Yoon T, Kim J, Kim J, Lee J (2013) Electrostatic self-assembly of Fe3O4 nanoparticles on graphene oxides for high capacity lithium-ion battery anodes. Energies 6:4830
Zhou J, Chen Q, Han Y, Zheng S (2015) Enhanced catalytic hydrodechlorination of 2,4-dichlorophenol over Pd catalysts supported on nitrogen-doped graphene. RSC Adv 5:91363–91371
Zhu Y, Murali S, Cai W, Li X, Suk JW, Potts JR, Ruoff RS (2010) Graphene and graphene oxide: synthesis, properties, and applications. Adv Mater 22:3906–3924
Acknowledgments
We are grateful to Kazunori Fujisawa, for technical assistance and useful discussions.
Funding
This work was supported by CTQ2015-65491_R and RTI2018-098431-B-I00 research grants. C. Ruiz-García also thanks for PhD and mobility grant (BES-2013-06608 5, EEBB-I-16-11722).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 389 kb)
Rights and permissions
About this article
Cite this article
Ruiz-Garcia, C., Lei, Y., Heras, F. et al. Functional Pd/reduced graphene oxide nanocomposites: effect of reduction degree and doping in hydrodechlorination catalytic activity. J Nanopart Res 21, 276 (2019). https://doi.org/10.1007/s11051-019-4696-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-019-4696-x