Abstract
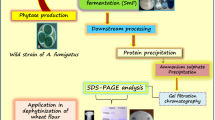
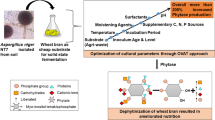
Phytases are the special class of enzymes which have excellent application potential for enhancing the quality of food by decreasing its inherent anti-nutrient components. In current study, a protease-resistant, acidic phytase from Aspergillus aculeatus APF1 was partially purified by ammonium sulfate fractionation followed by chromatography techniques. The molecular weight of partially purified phytase was in range of 25–35 kDa. The purified APF1 phytase was biochemically characterized and found catalytically active at pH 3.0 and 50 °C. The Km and Vmax values of APF1 phytase for calcium phytate were 3.21 mM and 3.78 U/mg protein, respectively. Variable activity was observed with metal ions and among inhibitors, chaotropic agents and organic solvents; phenyl glyoxal, potassium iodide, and butanol inhibited enzyme activity, respectively, while the enzyme activity was not majorly influenced by EDTA, urea, ethanol, and hexane. APF1 phytase treatment was found effective in dephytinization of flour biofortified wheat genotypes. Maximum decrease in phytic acid content was noticed in genotype MB-16-1-4 (89.98%) followed by PRH3–30-3 (82.32%) and PRH3–43-1 (81.47%). Overall, the study revealed that phytase from Aspergillus aculeatus APF1 could be effectively used in food and feed processing industry for enhancing nutritional value of food.





Similar content being viewed by others
References
Farouk, A., Banaja, A., Ahamed, N. T., AlZahrani, O., & Bazaid, S. (2015). Inducible secretion of phytate-degrading enzymes from bacteria associated with the medical plant Rosa damascena cv. Taifi using rice bran. African Journal of Biotechnology, 14, 425–433.
Lee, S. H., Cho, J., Bok, J., Kang, S., Choi, Y., & Lee, P. C. (2015). Characterization, gene cloning, and sequencing of a fungal phytase, PhyA, from Penicillium oxalicum PJ3. Preparative Biochemistry and Biotechnology, 45(4), 336–347.
Moreira, K. A., Herculano, P. N., Maciel, M., & Porto, T. S. (2014). Optimization of phytase production by Aspergillus japonicus Saito URM 5633 using cassava bast as substrate in solid state fermentation. African Journal of Microbiology Research, 8, 929–938.
Singh, B., & Satyanarayana, T. (2015). Fungal phytases: characteristics and amelioration of nutritional quality and growth of non-ruminants. Journal of Animal Physiology and Animal Nutrition, 99(4), 646–660.
Singh, B., Kunze, G., & Satyanarayana, T. (2011). Developments in biochemical aspects and biotechnological applications of microbial phytases. Biotechnology and Molecular Biology Reviews, 6, 69–87.
Silva, E. O., & Bracarense, A. P. F. (2016). Phytic acid: from antinutritional to multiple protection factor of organic systems. Journal of Food Science, 81, 1357–1362.
Holm, P. B., Kristiansen, K. N., & Pedersen, H. B. (2002). Transgenic approaches in commonly consumed cereals to improve iron and zinc content and bioavailability. The Journal of Nutrition, 132, 514–516.
Kumar, V., Sangwan, P., Verma, A., & Agrawal, S. (2014). Molecular and biochemical characteristics of recombinant β-propeller Phytase from Bacillus licheniformis strain PB-13 with potential application in aquafeed. Applied Biochemistry and Biotechnology, 173(2), 646–659.
Lei, X. G., Weaver, J. D., Mullaney, E., Ullah, A. H., & Azain, M. J. (2013). Phytase, a new life for an “old” enzyme. Annual Review on Animal Biosciences, 1, 283–309.
Gaind, S., & Singh, S. (2015). Production, purification and characterization of neutral phytase from thermotolerant Aspergillus flavus ITCC 6720. International Biodeterioration & Biodegradation, 99, 15–22.
Vohra, A., & Satyanarayana, T. (2003). Phytases: microbial sources, production, purification, and potential biotechnological applications. Critical Reviews in Biotechnology, 23(1), 29–60.
Fiske, C. H., & Subbarow, Y. (1925). The colorimetric determination of phosphorus. Journal of Biological Chemistry, 66, 375–400.
Singh, B., & Satyanarayana, T. (2009). Characterization of a HAP–phytase from a thermophilic mould Sporotrichum thermophile. Bioresource Technology, 100, 2046–2051.
Sapna, & Singh, B. (2017). Purification and characterization of a protease-resistant phytase of Aspergillus oryzae SBS50 whose properties make it exceptionally useful as a feed supplement. International Journal of Biological Macromolecules, 103, 458–466.
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72, 248–254.
Gao, Y., Shang, C., Maroof, M., Biyashev, R., Grabau, E., Kwanyuen, P., Burton, J., & Buss, G. (2007). A modified colorimetric method for phytic acid analysis in soybean. Crop Science, 47, 1797–1803.
Neira-Vielma, A. A., Aguilar, C. N., Ilyina, A., Contreras-Esquivel, J. C., Carneiro-da-Cunha, M. D. G., Michelena-Álvarez, G., & Martínez-Hernández, J. L. (2018). Purification and biochemical characterization of an Aspergillus niger phytase produced by solid-state fermentation using triticale residues as substrate. Biotechnology Reports, 17, 49–54.
Demir, Y., Şenol Kotan, M., Dikbaş, N., & Beydemir, Ş. (2017). Phytase from Weissella halotolerans: purification, partial characterisation and the effect of some metals. International Journal of Food Properties, 20, 2127–2137.
Dan, S. K., Nandi, A., Banerjee, G., Ghosh, P., & Ray, A. K. (2015). Purification and characterization of extracellular phytase from Bacillus licheniformis isolated from fish gut. Proceedings of the National Academy of Sciences, India Section B: Biological Sciences, 85, 751–758.
Li, M., Wang, H., & Bun Ng, T. (2013). Isolation of a phytase with distinctive characteristics from an edible mushroom. Pleurotus eryngii, Protein and Peptide Letters, 20(4), 459–466.
Segueilha, L., Lambrechts, C., Boze, H., Moulin, G., & Galzy, P. (1992). Purification and properties of the phytase from Schwanniomyces castellii. Journal of Fermentation and Bioengineering, 74, 7–11.
Sariyska, M., Gargova, S., Koleva, L., & Angelov, A. (2005). Aspergillus niger phytase: purification and characterization. Biotechnology & Biotechnological Equipment, 19, 98–105.
Gunashree, B., & Venkateswaran, G. (2015). Extracellular phytase from Aspergillus niger CFR 335: purification and characterization. Journal of Food Science and Technology, 52(7), 4558–4564.
Soni, S., Magdum, A., & Khire, J. (2010). Purification and characterization of two distinct acidic phytases with broad pH stability from Aspergillus niger NCIM 563. World Journal of Microbiology and Biotechnology, 26(11), 2009–2018.
Greiner, R., Silva, L. G. D., & Couri, S. (2009). Purification and characterization of an extracellular phytase from Aspergillus niger 11T53A9. Brazilian Journal of Microbiology, 40(4), 795–807.
Casey, A., & Walsh, G. (2003). Purification and characterization of extracellular phytase from Aspergillus niger ATCC 9142. Bioresource Technology, 86(2), 183–188.
Mullaney, E. J., & Ullah, A. H. (2003). The term phytase comprises several different classes of enzymes. Biochemical and Biophysical Research Communications, 312, 179–184.
Wyss, M., Pasamontes, L., Rémy, R., Kohler, J., Kusznir, E., Gadient, M., Müller, F., & van Loon, A. P. (1998). Comparison of the thermostability properties of three acid phosphatases from molds: Aspergillus fumigatus Phytase, A. niger Phytase, and A. niger pH 2.5 acid phosphatase. Applied and Environmental Microbiology, 64(11), 4446–4451.
Vats, P., & Banerjee, U. (2005). Biochemical characterisation of extracellular phytase (myo-inositol hexakisphosphate phosphohydrolase) from a hyper-producing strain of Aspergillus niger van Teighem. Journal of Industrial Microbiology and Biotechnology, 32(4), 141–147.
Azeke, M. A., Greiner, R., & Jany, K. D. (2011). Purification and characterization of two intracellular phytases from the tempeh fungus Rhizopus oligosporus. Journal of Food Biochemistry, 35, 213–227.
Bhavsar, K., Kumar, V. R., & Khire, J. (2011). High level phytase production by Aspergillus niger NCIM 563 in solid state culture: response surface optimization, up-scaling, and its partial characterization. Journal of Industrial Microbiology & Biotechnology, 38(9), 1407–1417.
Hesampour, A., Siadat, S. E. R., Malboobi, M. A., Mohandesi, N., Arab, S. S., & Ghahremanpour, M. M. (2015). Enhancement of thermostability and kinetic efficiency of Aspergillus niger PhyA phytase by site-directed mutagenesis. Applied Biochemistry and Biotechnology, 175(5), 2528–2541.
Jain, J., & Singh, B. (2016). Characteristics and biotechnological applications of bacterial phytases. Process Biochemistry, 51, 159–169.
Kumar, V., Yadav, A. N., Verma, P., Sangwan, P., Saxena, A., Kumar, K., & Singh, B. (2017). β-Propeller phytases: diversity, catalytic attributes, current developments and potential biotechnological applications. International Journal of Biological Macromolecules, 98, 595–609.
Ranjan, B., Singh, B., & Satyanarayana, T. (2015). Characteristics of recombinant phytase (rSt-Phy) of the thermophilic mold Sporotrichum thermophile and its applicability in dephytinizing foods. Applied Biochemistry and Biotechnology, 177(8), 1753–1766.
Tang, J., Leung, A., Leung, C., & Lim, B. L. (2006). Hydrolysis of precipitated phytate by three distinct families of phytases. Soil Biology and Biochemistry, 38, 1316–1324.
Patel, K. J., Singh, A. K., Nareshkumar, G., & Archana, G. (2010). Organic-acid-producing, phytate-mineralizing rhizobacteria and their effect on growth of pigeon pea (Cajanus cajan). Applied Soil Ecology, 44, 252–261.
Singh, B., Sharma, K., Kumari, A., Kumar, A., & Gakhar, S. (2018). Molecular modeling and docking of recombinant HAP-phytase of a thermophilic mould Sporotrichum thermophile reveals insights into molecular catalysis and biochemical properties. International Journal of Biological Macromolecules, 115, 501–508.
Gulati, H., Chadha, B., & Saini, H. (2007). Production, purification and characterization of thermostable phytase from thermophilic fungus Thermomyces lanuginosus TL-7. Acta Microbiologica et Immunologica Hungarica, 54(2), 121–138.
Zhang, G., Dong, X., Wang, Z., Zhang, Q., Wang, H., & Tong, J. (2010). Purification, characterization, and cloning of a novel phytase with low pH optimum and strong proteolysis resistance from Aspergillus ficuum NTG-23. Bioresource Technology, 101(11), 4125–4131.
Monteiro, P. S., Guimarães, V. M., Melo, R. R. D., & Rezende, S. T. D. (2015). Isolation of a thermostable acid phytase from Aspergillus niger UFV-1 with strong proteolysis resistance. Brazilian Journal of Microbiology, 46(1), 251–260.
Tripathi, P., Garg, S., Panwar, D., Kaira, G. S., Kumar, R., & Kapoor, M. (2017). Phytase from Citrobacter koseri PM-7: cost-effective production using agro-industrial residues. Biochemical Characterization and Application in de-Phytinization, Waste and biomass Valorization, 8, 1105–1118.
Díaz-Gómez, J., Twyman, R. M., Zhu, C., Farré, G., Serrano, J. C., Portero-Otin, M., Muñoz, P., Sandmann, G., Capell, T., & Christou, P. (2017). Biofortification of crops with nutrients: factors affecting utilization and storage. Current Opinion in Biotechnology, 44, 115–123.
Saltzman, A., Birol, E., Bouis, H. E., Boy, E., De Moura, F. F., Islam, Y., & Pfeiffer, W. H. (2013). Biofortification: progress toward a more nourishing future. Global Food Security, 2, 9–17.
Kalsi, H. K., Singh, R., Dhaliwal, H. S., & Kumar, V. (2016). Phytases from Enterobacter and Serratia species with desirable characteristics for food and feed applications, 3. Biotech, 6, 64.
Kaur, R., Saxena, A., Sangwan, P., Yadav, A. N., Kumar, V., & Dhaliwal, H. S. (2017). Production and characterization of a neutral phytase of Penicillium oxalicum EUFR-3 isolated from Himalayan region. Nusantara Bioscience, 9, 68–76.
Nielsen, A. V., & Meyer, A. S. (2016). Phytase mediated mineral solubilization from cereals under in vitro gastric conditions. Journal of the Science of Food and Agriculture, 96(11), 3755–3761.
Vashishth, A., Ram, S., & Beniwal, V. (2017). Cereal phytases and their importance in improvement of micronutrients bioavailability, 3. Biotech, 7, 42.
Gupta, R. K., Gangoliya, S. S., & Singh, N. K. (2015). Reduction of phytic acid and enhancement of bioavailable micronutrients in food grains. Journal of Food Science and Technology, 52(2), 676–684.
Weaver, C. M., & Kannan, S. (2002). Phytate and mineral bioavailability. Food Phytate, 211–223.
Nielsen, A., Tetens, I., & Meyer, A. (2013). Potential of phytase-mediated iron release from cereal-based foods: a quantitative view. Nutrients, 5(8), 3074–3098.
Akhter, S., Saeed, A., Irfan, M., & Malik, K. A. (2012). In vitro dephytinization and bioavailability of essential minerals in several wheat varieties. Journal of Cereal Science, 56, 741–746.
Hellström, A. M., Almgren, A., Carlsson, N. G., Svanberg, U., & Andlid, T. A. (2012). Degradation of phytate by Pichia kudriavzevii TY13 and Hanseniaspora guilliermondii TY14 in Tanzanian togwa. International Journal of Food Microbiology, 153(1-2), 73–77.
Sanz-Penella, J. M., Laparra, J. M. S., Sanz, Y., & Haros, M. (2012). Assessment of iron bioavailability in whole wheat bread by addition of phytase-producing bifidobacteria. Journal of Agricultural and Food Chemistry, 60(12), 3190–3195.
Bedford, M. R. (2000). Exogenous enzymes in monogastric nutrition—their current value and future benefits. Animal Feed Science and Technology, 86, 1–13.
Barrier-Guillot, B., Casado, P., Maupetit, P., Jondreville, C., Gatel, F., & Larbier, M. (1996). Wheat phosphorus availability: 2—in vivo study in broilers and pigs; relationship with endogenous phytasic activity and phytic phosphorus content in wheat. Journal of the Science of Food and Agriculture, 70, 69–74.
Qian, H., Kornegay, E., & Denbow, D. (1997). Utilization of phytate phosphorus and calcium as influenced by microbial phytase, cholecalciferol, and the calcium: total phosphorus ratio in broiler diets. Poultry Science, 76(1), 37–46.
Funding
Financial support was provided by the Department of Biotechnology (DBT), Govt. of India (Grant No. BT/AGR/BIOFORTI/PHII/NIN/2011) to carry out this work; Ministry of Food Processing Industries (MoFPI), Govt. of India, provided “infrastructural facility development” at Eternal University; and Head, Department of Microbiology, Maharshi Dayanand University, Rohtak permitted leading author to carry out part of this work at the Department.
Author information
Authors and Affiliations
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Saxena, A., Verma, M., Singh, B. et al. Characteristics of an Acidic Phytase from Aspergillus aculeatus APF1 for Dephytinization of Biofortified Wheat Genotypes. Appl Biochem Biotechnol 191, 679–694 (2020). https://doi.org/10.1007/s12010-019-03205-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-019-03205-9