Abstract
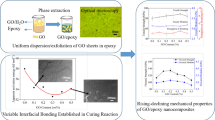
Liquid crystal (LC) monomer with Schiff base-grafted functionalized GO (M2-g-GO) sheets was successfully prepared to enhance the dispersion and interfacial interaction between additives (M2-g-GO) and the epoxy matrix (E-51), resulting in the enhancement of thermal and mechanical performances of epoxy nanocomposites. The structures and performances of neat epoxy, GO/epoxy nanocomposite, and M2-g-GO/epoxy nanocomposites were characterized by a few analytical tests, such as Fourier transform infrared spectra, X-ray diffraction, scanning electron microscopy, dynamic mechanical analyzer, differential scanning calorimetry, and thermogravimetric analysis. Meanwhile, the polarized optical microscope test displayed LC monomer with Schiff base-grafted GO has been successfully synthesized. The Tg and thermal performance of M2-g-GO/epoxy nanocomposites were distinctly promoted compared with those of the neat epoxy and GO/epoxy nanocomposite. Meanwhile, mechanical tests demonstrate the M2-g-GO/epoxy nanocomposites possess greater impact strength, flexural strength, and flexural modulus than those of the neat epoxy and GO/epoxy nanocomposite. It is also worth mentioning that the flexural strength with the adding content of 3 wt% increased by 62.1%, and the flexural modulus with the adding content of 3 wt% increased by 19.1%. Moreover, Td1% of M2-g-GO/epoxy nanocomposites were all higher than the neat epoxy (170 °C), and the Td5% of epoxy composites were around 385 °C, which were nearly 15 °C higher than that of neat epoxy. Meanwhile, the char yield of M2-g-GO/epoxy nanocomposites at 650 °C is 1.4% higher than that of 3 wt% GO/epoxy nanocomposites. Hence, the M2-g-GO exhibits predominant feasibility as effective increased thermal and toughness additives for leading to the enhancement of thermal and mechanical performances of nanocomposites.












Similar content being viewed by others
References
Lee JY, Jang JS (2006) The effect of mesogenic length on the curing behavior and properties of liquid crystalline epoxy resins. Polymer 47:3036–3042
Shen Y, Cong YH, Zhang BY, Lang QY, Hu B (2018) Dispersing nanoparticles by chiral liquid crystalline elastomers for performance enhancement of epoxy nanocomposites. Macromol Mater Eng 303:1–11
Chen F, Cong YH, Zhang BY (2016) Synthesis and characterization of liquid crystalline epoxy and co-polymerisation with a non-mesomorphic epoxy resin. Liq Cryst 43:1100–1109
Chen J, Taylor AC (2012) Epoxy modified with triblock copolymers: morphology, mechanical properties and fracture mechanisms. J Mater Sci 47:4546–4560. https://doi.org/10.1007/s10853-012-6313-6
Li XD, Xia YQ, Xu WL, Ran QC, Gu Y (2012) The curing procedure for a benzoxazine-cyanate-epoxy system and the properties of the terpolymer. Polym Chem 3:1629–1633
Wang X, Li N, Wang JY, Li GY, Zong LC, Liu C, Jian XG (2018) Hyperbranched polyether epoxy grafted graphene oxide for benzoxazine composites: enhancement of mechanical and thermal properties. Compos Sci Technol 155:11–21
Zhang ZE, Cai JC, Chen F, Li H, Zhang WX, Qi WJ (2018) Progress in enhancement of CO2 absorption by nanofluids: a mini review of mechanisms and current status. Renew Energy 118:527–535
Mahian O, Koisi L, Amani M, Estelle P, Ahmadi G, Kleinstreuer C, Marshall JS, Siavashi M, Tayior RA, Niazmand H, Wongwises S, Hayat T, Kolanjiyil A, Kasaeian A, Pop I (2019) Recent advances in modeling and simulation of nanofluid flows—part I: fundamentals and theory. Phys Rep 790:1–48
Liu K, Lu SR, Li SR, Huang B, Wei C (2012) Mechanical and thermal properties of POSS-g-GO reinforced epoxy composites. Iran Polym J 21:497–503
Lu SR, Li SR, Yu JH, Yuan ZK, Qi B (2013) Epoxy nanocomposites filled with thermotropic liquid crystalline epoxy grafted graphene oxide. RSC Adv 38:8915–8923
Lee CH, Yang CK (2012) Coupling of a carbon nanotube and graphene nanoribbon by titanium and vanadium chains: a first-principles study. RSC Adv 2:9958–9963
Shau SM, Juang TY, Lin HS, Huang CL, Hsieh CF, Wu JY, Jeng RJ (2012) Individual graphene oxide platelets through direct molecular exfoliation with globular amphiphilic hyperbranched polymers. Polym Chem 3:1249–1259
Liu XJ, Pan LK, Lv T, Zhu G, Lu T, Sun Z, Sun CQ (2011) Microwave-assisted synthesis of TiO2-reduced graphene oxide composites for the photocatalytic reduction of Cr(VI). RSC Adv 1:1245–1249
Cheng HKF, Sahoo NG, Tan YP, Pan YZ, Bao HQ, Li L, Chan SH, Zhao JH (2012) Poly(vinyl alcohol) nanocomposites filled with poly(vinyl alcohol)-grafted graphene oxide. ACS Appl Mater Interfaces 4:2387–2394
Paszkiewicz S, Szymczyk A, Pilawka R, Przybyszewski B, Czulak A, Roslaniec Z (2017) Improved thermal conductivity of poly(trimethylene terephthalate-block-poly(tetramethylene oxide)) based nanocomposites containing hybrid single-walled carbon nanotubes/graphene nanoplatelets fillers. Adv Polym Technol 36:236–242
Limbu TB, Hahn KR, Mendoza F, Sahoo S, Razink JJ, Katiyar RS, Weiner BR, Morell G (2017) Grain size-dependent thermal conductivity of poly-crystalline twisted bilayer graphene. Carbon 117:367–375
Rafifiee MA, Rafifiee J, Srivastava I, Wang Z, Song HH, Yu ZZ, Koratkar N (2010) Fracture and fatigue in graphene nanocomposites. Small 6:179–183
Men YJ, Li XH, Antonietti M, Yuan JY (2012) Poly(tetrabutylphosphonium 4-styrenesulfonate): a poly(ionic liquid) stabilizer for graphene being multi-responsive. Polym Chem 3:871–873
Lei L, Shan J, Hu J, Liu X, Zhao J, Tong Z (2016) Co-curing effect of imidazole grafting graphene oxide synthesized by one-pot method to reinforce epoxy nanocomposites. Compos Sci Technol 128:161–168
Dai Z, Wang G, Liu L, Hou Y, Wei Y, Zhang Z (2016) Mechanical behavior and properties of hydrogen bonded graphene/polymer nano-interfaces. Compos Sci Technol 136:1–9
Paredes JI, Villar-Rodil S, Martinez-Alonso A, Tascon JMD (2008) Graphene oxide dispersions in organic solvents. Langmuir ACS J Surf Colloids 24:10560–10564
Shen B, Zhai W, Tao M, Lu D, Zheng W (2013) Chemical functionalization of graphene oxide toward the tailoring of the interface in polymer composites. Compos Sci Technol 77:87–94
Takahashi A, Lin CJ, Ohshimizu K, Higashihara T, Chen WC, Ueda M (2012) Synthesis and characterization of novel polythiophenes with graphene-like structures via intramolecular oxidative coupling. Polym Chem 3:479–485
Bai S, Shen XP (2012) Graphene-inorganic nanocomposites. RSC Adv 2:64–98
Dreyer DR, Jarvis KA, Ferreira PJ, Bielawski CW (2012) Graphite oxide as a carbocatalyst for the preparation of fullerene-reinforced polyester and polyamidenanocomposites. Polym Chem 3:757–766
Ma YX, Li YF, Zhao GH, Yang LQ, Wang JZ, Shan X, Yan X (2012) Preparation and characterization of graphite nanosheets decorated with Fe3O4 nanoparticles used in the immobilization of glucoamylase. Carbon 50:2976–2986
Ma D, Lin JT, Chen YY, Xue W, Zhang LM (2012) In situ gelation and sustained release of an antitumor drug by graphene oxide nanosheets. Carbon 50:3001–3007
Zhang XD, Cong YH, Zhang BY (2017) Reduced graphene oxide/liquid crystalline oligomer composites based on reversible covalent chemistry. Phys Chem Chem Phys 19:6082–6089
Henmi M, Nakatsuji K, Ichikawa T, Tomioka H, Sakamoto T, Yoshio M, Kato T (2012) Self-organized liquid-crystalline nanostructured membranes for water treatment: selective permeation of ions. Adv Mater 24:2238–2241
Lin PC, Cong YH, Sun C, Zhang BY (2016) Non-covalent modification of reduced graphene oxide by a chiral liquid crystalline surfactant. Nanoscale 8:2403–2411
Zhao GX, Wen T, Chen CL, Wang XK (2012) Synthesis of graphene-based nanomaterials and their application in energy-related and environmental-related areas. RSC Adv 2:9286–9303
Guo HL, Peng M, Zhu ZM, Sun LN (2013) Preparation of reduced graphene oxide by infrared irradiation induced photo-thermal reduction. Nanoscale 5:9040–9048
Zhu YW, Murali S, Cai WW, Li XS, Suk JW, Potts JR, Ruoff RS (2010) Synthesis, properties, and applications. Adv Mater 22:3906–3924
Seo JM, Baek JB (2014) A solvent-free Diels-Alder reaction of graphite into functionalized graphene nanosheets. Chem Commun 50:14651–14653
Yuan J, Chen G, Weng W, Xu Y (2012) One-step functionalization of graphene with cyclopentadienyl-capped macromolecules via Diels-Alder “click” chemistry. J Mater Chem 22:7929–7936
Li J, Zhang G, Deng L, Zhao S, Gao Y, Jiang K, Sun R, Wong C (2014) In situ polymerization of mechanically reinforced, thermally healable graphene oxide/polyurethane composites based on Diels-Alder chemistry. J Mater Chem A 2:20642–20649
Seo JM, Jeon IY, Baek JB (2013) Mechanochemically driven solid-state Diels-Alder reaction of graphite into graphene nanoplatelets. Chem Sci 4:4273–4277
Su YG, Tang J, Zhang K, Yuan JS, Li J, Zhu DM, Ozawa K, Qin LC (2017) Comparison of reduction products from graphite oxide and graphene oxide for anode applications in lithium-ion batteries and sodium-ion batteries. Nanoscale 9:2585–2595
Yan YN, Kuila T, Kim NH, Ku B-C, Lee JH (2013) Effects of reduction and polystyrene sulfate functionalization on the capacitive behaviour of thermally exfoliated graphene. J Mater Chem A 1:5892–5901
Krishnamoorthy K, Veerapandian M, Yun K, Kim SJ (2013) The chemical and structural analysis of graphene oxide with different degrees of oxidation. Carbon 53:38–49
Yang DX, Velamakanni A, Bozoklu G, Park S, Stoller M, Piner RD, Stankovich S, Jung I, Field DA, Ventrice CA, Ruoff RS (2009) Chemical analysis of graphene oxide films after heat and chemical treatments by X-ray photoelectron and micro-Raman spectroscopy. Carbon 47:145–152
Hu YZ, Shen JF, Li N, Shi M, Ma HW, Yan B, Wang WB, Huang WS, Ye MX (2010) Amino-functionalization of graphene sheets and the fabrication of their nanocomposites. Polym Compos 31:1987–1994
Allen MJ, Tung VC, Kaner RB (2010) Honeycomb carbon: a review of graphene. Chem Rev 110:132–145
Yu JH, Huang XY, Wu C, Wu XF, Wang GL, Jiang PK (2012) Interfacial modification of boron nitride nanoplatelets for epoxy composites with improved thermal properties. Polymer 53:471–480
Wan YJ, Tang LC, Gong LX, Yan D, Li YB, Wu LB, Jiang JX, Lai GQ (2014) Grafting of epoxy chains onto graphene oxide for epoxy composites with improved mechanical and thermal properties. Carbon 69:467–480
Guan LZ, Wan YJ, Gong LX, Yan D, Tang LC, Wu LB, Jiang JX, Lai GQ (2014) Toward effective and tunable interphases in graphene oxide/epoxy composites by grafting different chain lengths of polyetheramine onto graphene oxide. J Mater Chem A 2:15058–15069
Wang X, Jin J, Song M (2013) An investigation of the mechanism of graphene toughening epoxy. Carbon 65:324–333
Zaman I, Phan TT, Kuan HC, Meng Q, La LTB, Luong L, Youssf O, Ma J (2011) epoxy/graphene platelets nano-composites with two levels of interface strength. Polymer 52:1603–1611
Park YT, Qian Y, Chan C, Suh T, Nejhad MG, Macosko CW, Stein A (2015) epoxy toughening with low graphene loading. Adv Funct Mater 25:575–585
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. U1708258).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hu, B., Cong, Yh., Zhang, By. et al. Enhancement of thermal and mechanical performances of epoxy nanocomposite materials based on graphene oxide grafted by liquid crystalline monomer with Schiff base. J Mater Sci 55, 3712–3727 (2020). https://doi.org/10.1007/s10853-019-04273-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-019-04273-2