Abstract
The copper coordination complex [CuCl(2-OHNA)H2O]·H2O (1) was synthesized by the reaction of CuCl2·2H2O with in situ generated 2-hydroxynicotinic acid and its crystal structure was determined by single X-ray diffraction methods. It was further characterized by FT-IR spectroscopy, elemental analyses, and thermogravimetric analysis. Complex 1 crystallizes in monoclinic space group P21/c with a = 8.9797(13), b = 14.196(2), c = 7.0738(11) Å, β = 96.897(2), V = 895.2(2) Å3, Mr = 273.12, Dc = 2.027 g/cm3, and Z = 4. In the structure, complex 1 is linked into 2D sheets via intermolecular hydrogen bonding [N1–H1···O2 (−x+1, y − 1/2, − z+1/2); O5–H10···O2 (− x+1, y + 1/2, − z+1/2); O4–H9···O5 (x, y − 1, z); O4–H8···O5 (− x+2, y + 1, − z)]. The catalytic property of 1 was also investigated in the selective oxidation of benzyl-alkanes using TBHP as oxidant. Under optimized conditions, 1 exhibited high catalytic activity and selectivity toward the corresponding aryl ketones.
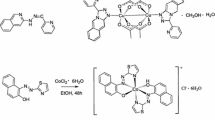
Graphic Abstract
The copper coordination complex [CuCl(2-OHNA)H2O]·H2O (1) was synthesized by the reaction of CuCl2·2H2O with in situ generated 2-hydroxynicotinic acid and the catalytic property of 1 was also investigated in the selective oxidation of benzyl-alkanes using TBHP as oxidant.

Similar content being viewed by others
Introduction
In recent decades, transition-metal complexes have obtained long-lasting attention due to their variety of architectures and the potential applications as functional materials [1,2,3,4]. Among transition metals, copper is an important element in catalysis chemistry, coordination chemistry, magnetochemistry and its relevance as the active-site structure of metalloproteins [5,6,7,8]. Moreover, its flexible coordination modes make the copper ion to form mononuclear, dinuclear and multinuclear complexes. Functionalized carboxylates can easily be applied to construct functionalized metal–organic coordination compounds with supramolecular architectures. Functional groups like –OH, –NH2 are frequently used for this purpose [9]. 2-Hydroxynicotinic acid (2-OHNAH) is one of the most successful functionalized carboxylates due to its versatile modes of coordination [10].
Herein, we present a Cu(II) coordination complex [CuCl(2-OHNA)H2O]·H2O (1) with in situ generated 2-hydroxynicotinic acid. Complex 1 was fully characterized by single crystal X-ray diffraction (SXRD), FT-IR spectroscopy, elemental analysis (EA), and thermogravimetric (TGA) analysis. The metal contents of 1 were determined using inductively coupled plasma (ICP) on a JY-ULTIMA2 analyzer. Complex 1 was obtained by mixing CuCl2·2H2O and 2-chloronicotinic acid (2-ClNAH) in 1:2 molar ratio in a 1:2 water–methanol mixture under basic conditions. In 1, the original 2-ClNAH ligand underwent to a base hydrolysis reaction followed by keto-enol tautomerization (Scheme 1). Importantly, complex 1 was further investigated as catalyst for the oxidation of benzylic sp3 C–H bonds with TBHP as an oxidant.
Experimental
All reagents and solvents were purchased from commercial sources and used without further purification. The C, H and N elemental analyses were conducted on a Perkin-Elmer 240C elemental analyzer. The FT-IR spectra were recorded from KBr pellets in the range of 4000–400 cm−1 on a Nicolet 170 SXFT/IR spectrometer. The GC analyses were performed on Shimadzu GC-2014C with a FID detector equipped with an Rtx-1701 Sil capillary column. TGA experiments were carried out on a Perkin-Elmer TGA 7 analyzer with a heating rate of 10 °C/min from room temperature to 700 °C under nitrogen atmosphere.
Synthesis of Complex [CuCl(2-OHNA)H2O]·H2O (1)
To a 40 mL methanolic solution of 2-ClNAH (0.95 g, 6 mmol) was slowly added a 20 mL aqueous solution of CuCl2·2H2O (0.52 g, 3 mmol) with continuous stirring. The mixture was stirred for 1 h with constant heating at 60 °C. Subsequently, the solution was allowed to cool and the pH was adjusted to about 7.5 with an aqueous solution of NaOH. The mixture was then heated over a water bath for 2 h and subsequently filtered. The solution was left to over a week at room temperature to evaporate the solvent and until blue colored translucent crystals formed (ca. 52% yield based on 2-ClNAH). Anal. calcd. (%) for C6H8ClCuNO5: C, 26.38; H, 2.95; N, 5.13. Found: C, 26.24; H, 3.11; N, 5.06. IR (KBr) v: 3442, 3338, 1664, 1616, 1552, 1473, 1247, 1148, 1092, 672, 577, 542, 441 cm−1.
X-ray Crystallography
Single-crystal X-ray diffraction data for complex 1 were collected on a Bruker Smart APEX II CCD diffractometer using graphite-monochromated Mo Ka radiation (λ = 0.71073 Å) at 298 K. The data were corrected for absorption using the multi-scan technique [11]. The structure was solved by the direct method and refined through full-matrix least-squares techniques method on F2 using the SHELXTL 97 crystallographic software package [12, 13]. The hydrogen atoms connected to C and N atoms were placed in calculated positions and refined using a riding model. Hydrogen atoms on O atoms were located in the difference Fourier maps and then refined using a riding model and a dependent displacement parameter Uiso(H) = 1.5Ueq(O). The distances of O–H bonds and bond angles of H–O–H were further restrained using the DFIX command. Crystallographic data for complex 1 are summarized in Table 1 and selected bond lengths and bond angles are listed in Table 2.
Catalytic Oxidation of Benzylic sp3 C–H Bonds
In a typical experiment, the required amount of catalyst (1.5 mol%) and benzyl hydrocarbon (0.125 mmol), 70% TBHP (0.35 mmol) and benzonitrile (1 mL) were successively added to a round-bottom flask. The reaction mixture was subsequently heated at 80 °C in a Wattecs Parallel Reactor for 24 h with stirring. The conversion and selectivity of these reactions were determined by GC analyses with nitrobenzene as an internal standard. All of the products were confirmed by GC analysis in combination with GC–MS.
Results and Discussion
Thermogravimetric Analysis of [CuCl(2-OHNA)H2O]·H2O (1)
Thermogravimetric analysis (TGA) was performed to study the stability of complex 1 (Fig. 1). Three separate weight loss steps were observed: The first weight loss began at 90 °C and was completed at 160 °C. The observed weight loss of 19.90% corresponds to the loss of one lattice water molecule and –Cl− group (calcd 19.95%). The second weight loss of 24.72% occurred in the range of 310–540 °C, corresponding to the loss of one coordinated water molecule and one –COO− group (calcd 24.17%). The third and continuous weight loss step began at 543 °C and can be attributed to the further decomposition of the 2-OHNA ligand.
Crystal Structure of [CuCl(2-OHNA)H2O]·H2O (1)
Compound 1 including the hydrogen-bonded solvent water is shown in Fig. 2. SXRD analysis shows that complex 1 crystallizes in the monoclinic space group P21/c. The Cu2+ ion is square planar coordination as expected. Chlorine atoms in the neighboring sheet are at a 2.80 Å distance from Cu. The ligand 2-OHNA exists in keto form and bidentately coordinates to the metal centre in O, O′-salicylate manner. The typical square planar coordination around the Cu(II) ion includes three O atoms (two from the 2-OHNA ligand and one from the coordinated water molecule) and one Cl atom. The chelate bite angle in the five-membered ring formed by O1 (carboxyl group) and O3 (carbonyl group) is 92.9°. The distances of Cu1–O1 and Cu1–O3 are 1.920(7) Å and 1.914(9) Å respectively, which are all considerably shorter than the Cu1–O4 (1.937(1) Å) distance indicating that the coordination bond between copper and water molecule (O4) is relatively weaker than the chelate bonds between copper and the 2-OHNA ligand (O1 and O3). The bond lengths and angles between the nicotinic acid and Cu are comparable with structurally characterized complexes previously reported [14,15,16].
Further, hydrogen bondings are observed as weak supramolecular interactions to stabilize the lattice structure (Fig. 3). First, the adjacent mononuclear structures are connected by the hydrogen bonding interaction formed by N1 of the pyridine ring and O2 derived from the carboxyl group of the adjacent 2-OHNA anion with the N1–H1···O2 (− x + 1, y − 1/2, − z + 1/2) distance of 2.825(2) Å to form a 1D supramolecular chain. Second, the solvent water molecules along with the coordinated water molecules connect the neighboring 1D chains to generate 2D layers by the hydrogen bonding interactions (O5–H10···O2 (− x + 1, y + 1/2, − z +1 /2) = 2.798(2) Å, O4 − H9···O5 (x, y − 1, z) = 2.732(2) Å, O4–H8···O5 (− x + 2, y + 1, − z) = 2.758(2) Å). At last, the 2D layers are stacked by solvent water hydrogen-bonding to Cl to form a 3D supramolecular structure with the O5–H11···Cl1 (− x + 2, y + 1/2, − z + 1/2) distance of 3.193(6) Å. All hydrogen bonds are shown in Table 3.
Catalytic Activity of [CuCl(2-OHNA)H2O]·H2O (1)
Selective oxidation of benzyl-alkanes is a powerful tool to generate high value organic compounds from less expensive raw materials and represents one of the formidable challenges in organic chemistry [17, 18]. However, a few catalysts have shown catalytic activity in the oxidation of benzylic C–H bonds with unsatisfactory catalytic activity [19,20,21]. With the aim to understand the catalytic property of the title coordination polymer for developing a new oxidation system in organic chemistry, the oxidation of diphenylmethane to benzophenone was selected as a model reaction (Scheme 2). While molecular oxygen and hydrogen peroxide were inefficient to promote diphenylmethane oxidation, TBHP was a good oxidizing reagent in terms of the percentage yield of benzophenone. Thus, TBHP was chosen as the best oxidant in the reaction. After the preliminary optimization, benzophenone is obtained as the almost sole product in 97.5% conversion using TBHP as oxidant in the presence of complex 1 at 80 °C for 24 h. And indeed, the catalytical activity of complex 1 outperforms those of some transition-metal-based complexes reported to date, i.e. [Cu4L4]4X (L = 1,3,5-tris(1-benzylbenzimidazol-2-yl)benzene, X = ClO4, OTs, OTf) [22], NHPI/Cu@PILC [23].
The excellent performance in the transformation of diphenylmethane to benzophenone prompted us to extend the scope of complex 1 as catalyst for other benzylic sp3 C–H bonds. As illustrated in Table 4, complex 1 exhibits excellent catalytic activity for oxidation of benzyl sp3 C–H bonds, fluorene and its derivates, 9H-xanthenes are all oxidized to the corresponding aryl ketones with great conversion rate (98.5–99.6%) and exceptional selectivity (97.5–99.5%). That is to say, complex 1 could facilitate the oxidation of benzylic sp3 C–H bonds and may serve as highly efficient and selective catalyst.
Conclusion
In summary, we have synthesized a copper coordination complex [CuCl(2-OHNA)H2O]·H2O (1) by the reaction of CuCl2·2H2O with in situ generated 2-hydroxynicotinic acid. The introduction of Cu2+ cation make complex 1 more stable and can be used as catalysts in the selective oxidation of alkylbenzenes with high catalytic activity and high selectivity toward the corresponding aryl ketones. Intramolecular hydrogen-bonds help to form a 2D layers. Complex 1 shows excellent generality in converting benzylic sp3 C–H bonds compounds to corresponding aromatic ketones efficiently. Investigations on the use of this complex for other potential catalytic reactions are in progress.
Supplementary Material
CCDC 1569265 contains the supplementary crystallographic data for this paper. This data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif, by e-mailing data_request@ccdc.cam.ac.uk.
References
Zhang JP, Zhang YB, Lin JB, Chen XM (2012) Chem Rev 112:1001
Yin Y, Tan Z, Hu L, Yu S, Liu J, Jiang G (2017) Chem Rev 117:4462
Paskevicius M, Jepsen LH, Schouwink P, Černý R, Ravnsbæk DB, Filinchuk Y, Dornheim M, Besenbacher F, Jensen TR (2017) Chem Soc Rev 46:1565
Zhao Y, Li Z, Sharma N, Song G, Eycken EVV (2016) Chem Commun 52:6395
Teong SP, Yu D, Sum YN, Zhang Y (2016) Green Chem 18:3499
Tirsoaga A, Cojocaru B, Teodorescu C, Vasiliu F, Grecu MN, Ghica D, Parvulescu VI, Garcia H (2016) J Catal 341:205
Solomon EI, Randall DW, Glaser T (2000) Coord Chem Rev 200:595
Cernak J, Orendac M, Potocnak I, Chomic J, Orendacova A, Skorsepa J, Feher A (2002) Coord Chem Rev 224:51
Ma M, Noei H, Mienert B, Niesel J, Bill E, Muhler M, Fischer RA, Wang Y, Schatzschneider U, Metzler-Nolte N (2013) Chem Eur J 19:6785
Singh SK, Srivastava AK, Srivastava K, Banerjee R, Prasad J (2017) J Mol Struct 1147:549
Sheldrick GM (1996) SADABS. Göttingen University, Germany
Sheldrick GM (2015) Acta Cryst A 71:3
Sheldrick GM (2015) Acta Cryst C 71:3
Lü Y, Zhang X, Cui XB, Xu JQ (2018) Inorg Chem 57:11123
Hu YY, Zhang TT, Zhang X, Zhao DC, Cui XB, Huo QS, Xu JQ (2016) Dalton Trans 45:2562
Zhao DC, Hu YY, Ding H, Guo HY, Cui XB, Zhang X, Huo QS, Xu JQ (2015) Dalton Trans 44:8971
Giri R, Shi BF, Engle KM, Maugel N, Yu JQ (2009) Chem Soc Rev 38:3242
Díaz-Requejo MM, Pérez PJ (2008) Chem Rev 108:3379
Chughtai AH, Ahmad N, Younus HA, Laypkov A, Verpoort F (2015) Chem Soc Rev 44:6804
Zhao M, Ou S, Wu CD (2014) Acc Chem Res 47:1199
Chen YF, Huang XQ, Feng X, Li JK, Huang YY, Zhao JS, Guo YX, Dong XM, Han RD, Qi PF, Han YZ, Li HW, Hu CW, Wang B (2014) Chem Commun 50:8374
He QT, Li XP, Chen LF, Zhang L, Wang W, Su CY (2013) ACS Catal 3:1
Zhao Q, Zhang P, Antonietti M, Yuan J (2012) J Am Chem Soc 134:11852
Acknowledgements
This work was financially supported by the Natural Science Foundation of Shandong Province (ZR2017LB002) and project (2018HX207), Taian Municipal Science and Technology Project (2015GX2057) and the Talent Introduction Project of Taishan University (Y2015-1-009).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wu, RT., Li, JK., Wei, CP. et al. Synthesis, Crystal Structure, and Catalytic Property of a Copper Coordination Compound Based on In Situ Generated 2-Hydroxynicotinic Acid. J Chem Crystallogr 50, 234–240 (2020). https://doi.org/10.1007/s10870-019-00818-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10870-019-00818-0