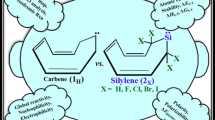
Abstract
In contrast to cyclonona-3,5,7-trienylidene (1H) which turns out as a boat-shaped transition state for having a negative force constant, its heavier plumbylenic analogous (2X) where X = H, F, Cl, Br, and I emerge as boat-shaped minima. This unsubstituted carbene has a triplet ground state while exclusive of 2I which initially takes on a triplet multiplicity and eventually transforms to a less stable intramolecular ring opening product; all of the plumbylenes (2H, 2F, 2Cl, and 2Br) have a singlet ground state. Hence, stability anticipated by the singlet (S)–triplet (T) splitting (ΔES-T) decreases by going down in the group 17 column: 2Br > 2Cl > 2F > 2H > 1H > 2I. Also, the HOMO-LUMO gap (ΔEHOMO-LUMO) increases as a result of substituting. From a thermodynamic perspective, our scrutinized 2Br, 2Cl, and 2F are found 1.5–2 times more stable than that of the reported cyclopenta-2,4-dienplumbylene and 2,5-bis(halobora)-cyclopentenplumbylenes analogues, respectively. From a kinetic perspective, these nine-membered plumbylenes are found 20–26 kcal/mol more stable than that of their corresponding five-membered congeners. The NBO analysis on stable singlet 2Br shows that there is a mesomeric interaction between bromine lone-pair electron and the Pb divalent atom of 2Br with bonding (σ) and anti-bonding (σ*) orbitals of carbon–bromine and carbon–lead. The main stabilizing effect appears to be π- and σ-bond hyperconjugation among the iodine heteroatom and divalent center of triplet 2I. This research signifies the thermodynamic and kinetic stabilities of the biggest unsaturated cyclic plumbylenes hoping to prompt the experimental attention toward them.












Similar content being viewed by others
References
The Organometallic Chemistry of N-heterocyclic Carbenes, 1st edn. Han Vinh Huynh. © 2017 John Wiley & Sons Ltd. Published 2017.
Stander-Grobler E, Schuster O, Heydenrych G, Cronje S, Tosh E, Albrecht M, Frenking G, Raubenheimer HG (2010) Pyridine-derived N-heterocyclic carbenes: an experimental and theoretical evaluation of the bonding in and reactivity of selected normal and abnormal complexes of nickel(II) and palladium(II). Organomet 29:5821
Arduengo III AJ, Harlow RL, Kline M (1991) A stable crystalline carbene. J Am Chem Soc 113:361
Arduengo III AJ, Dias HVR, Harlow RL, Kline M (1992) Electronic stabilization of nucleophilic carbenes. J Am Chem Soc 114:5530
Wu C-S, Su M-D (2012) Reactivity for boryl(phosphino)carbenyl carbene analogues with group 14 elements (C, Si, Ge, Sb, and Pb) as a heteroatom: a theoretical study. Dalton Trans 41:3253
Dickschat JV, Heitmann D, Pape T, Hahn FE (2013) Synthesis of stable diphenyl-di(germylene) and diphenyldi(plumbylene). J Organomet Chem 744:160
Davidson PJ, Lappert MF (1973) Stabilisation of metals in a low co-ordinative environment using the bis(trimethylsilyl)methyl ligand; coloured SnII and PbII alkyls, M[CH(SiMe3)2]2. J Chem Soc Chem Commun 317
Harris DH, Lappert MF (1974) Monomeric, volatile bivalent amides of group IVB elements, M(NR12)2 and M(NR1R2)2(M=Ge, Sn, or Pb; R1=Me3Si, R2=Me3C). J Chem Soc Chem Commun 895
Fjeldberg T, Hope H, Lappert MF, Power PP, Thorne AJ (1983) Molecular structures of the main group 4 metal(II) bis(trimethylsilyl)-amides M[N(SiMe3)2]2 in the crystal (X-ray) and vapour (gas-phase electron diffraction). J Chem Soc Chem Commun 639
Braunschweig H, Gehrhus B, Hitchcock PB, Lappert MF (1992) The first monomeric prochiral tin(II) complexes Sn[N(SiMe3)2]X [X = OC6H2But2-2,6-Me-4, 1 or NCMe2(CH2)3CME2, 2]; the X-ray structure of 1 and oxidative addition reactions of 2. J Chem Soc Chem Commun 1311
Denk M, Lennon JR, Hayashi R, West R, Belyakov AV, Verne HP, Haaland A, Wagner M, Metzler N (1994) Synthesis and structure of a stable silylene. J Am Chem Soc 116:2691
Veith M (1987) Unsaturated molecules containing main group metals. Angew Chem Int Ed Eng 26:1
Balasubramanian K, McLean AD (1986) The singlet–triplet energy separation in silylene. J Chem Phys 85:5117
Balasubramanian K (1988) Relativistic configuration interaction calculations for polyatomics: applications to PbH2, SnH2, and GeH2. J Chem Phys 89:5731
Lloyd D, Peterson NW (1966) Diazocyclononatetraene. Chem Ind 1039
Waali EE, Taylor JL, Allison NT (1977) On the isolation of diazocyclononatetraene. Tetrahedron Lett 44:4873
Waali EE, Wright CW (1981) Cyclononatetraenylidene. J Organomet Chem 46:2201
Glidewell C, Lloyd D (1983) Cyclo-C9H8, -C7H6, and C5H4: are they allenes, carbenes, or onium anions or carbanion cations. J Chem Res (S) 139:178
Kassaee MZ, Nimlos MR, Downie KE, Waali EE (1985) A mndo study of 3-, 5-, 7-and 9-membered carbocyclic, completely conjugated, planar carbenes and their nonplanar isomers. Tetrahedron 41:1579
Petrov AA, Federova AV (1964) Allene hydrocarbons. Russ Chem Rev (Engl Transl) 33:1
Kassaee MZ (1985) PhD Thesis in organic chemistry (reactive intermediates), generation and characterization of conjugated monocyclic C9H8 carbenes. University of Montana, Missoula, MT, USA
Kassaee MZ, Koohi M (2005) Mirror image conversions of cyclic conjugated non-planar allenes, C9H7X (X=H, F, Cl, Br). J Mol Struct (THEOCHEM) 755:91
Kassaee MZ, Koohi M, Arshadi S (2005). J Mol Struct (THEOCHEM) 724:61
Kassaee MZ, Koohi M (2007) Ring flips of allenes (C9H7X) over triplet carbenes at ab initio and DFT levels (X = H, F, Cl, Br). J Mol Struct (THEOCHEM) 815:21
Kassaee MZ, Koohi M (2007) Umbrella inversions of cyclononatetraenylidenes at ab initio and DFT. J Mol Struct (THEOCHEM) 810:53
Kassaee MZ, Koohi M (2005) Beyond C9H8 carbene mechanisms via13C-NMR and 13C- labeling, Pittsburgh Conference (Pittcon), Orlando, Florida, USA, Feb. 27-March 4; Book of Abstracts
Kassaee MZ, Bekhradnia AR, Talebzadeh S (2005) Isotope effects by comparing 1H & 2D NMR spectra of 9,9’- bisbicyclo[4.3.0] cyclo-nona-2,4,7-triene. Spectrosc Lett 38:487
Koohi M, Kassaee MZ, Haerizade BN, Ghavami M, Ashenagar S (2015) Substituent effects on cyclonona-3,5,7-trienylidenes: a quest for stable carbenes at density functional theory level. J Phys Org Chem 28:514
Kassaee MZ, Koohi M (2013) Breathing viability into cyclonona-3,5,7-trienylidenes via α-dimethyl and ά-moieties at DFT. J Phys Org Chem 26:540
Kassaee MZ, Koohi M, Mohammadi R, Ghavami M (2013) 2,2,9,9-Tetramethylcyclonona-3,5,7-trienylidene vs. its heterocyclic analogues: a quest for stable carbenes at DFT. J Phys Org Chem 26:908
Kassaee MZ, Momeni MR, Shakib FA, Ghambarian M, Musavi SM (2010) Novel α-spirocyclic (alkyl) (amino) carbines at the theoretical crossroad of flexibility and rigidity. Struct Chem 21:593
Kassaee MZ, Shakib FA, Momeni MR, Ghambarian M, Musavi SM (2010) Carbenes with reduced heteroatom stabilization: a computational approach. J Organomet Chem 75:2539
Kassaee MZ, Ghambarian M, Musavi SM, Shakib FA, Momeni MR (2009) A theoretical investigation into dimethylcarbene and its diamino and diphosphino analogs: effects of cyclization and unsaturation on the stability and multiplicity. J Phys Org Chem 22:919
Kassaee MZ, Shakib FA, Momeni MR, Ghambarian M, Musavi SM (2009) A DFT study on pyridine-derived N-heterocyclic carbenes. Tetrahedron 65:10093
Schmidt MW, Baldridge KK, Boatz JA, Elbert ST, Gordon MS, Jensen JH, Koseki S, Matsunaga N, Nguyen KA, Su SJ, Windus TL, Dupuis M, Montgomery JA (1993) General atomic and molecular electronic structure system. J Comput Chem 14(11):1347
Sobolewski AL, Domcke W (2002) Ab initio investigation of the structure and spectroscopy of hydronium−water clusters. J Phys Chem A 106:4158
Becke AD (1988) Density-functional exchange-energy approximation with correct asymptotic behavior. Phys Rev A 38:3098
Becke AD (1993) Density-functional thermochemistry. III. The role of exact exchange. J Chem Phys 98:5648
Becke AD (1996) Density-functional thermochemistry. IV. A new dynamical correlation functional and implications for exact-exchange mixing. J Chem Phys 104:1040
Lee C, Yang W, Parr RG (1988) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37:785
Krishna R, Frisch MJ, Pople JA (1980) Contribution of triple substitutions to the electron correlation energy in fourth order perturbation theory. J Chem Phys 72:4244
Francl MM, Pietro WJ, Hehre WJ, Binkley JS, Gordon MS, DeFrees DJ, Pople JA (1982) Self-Consistent Molecular Orbital Methods. XXIII. A polarization-type basis set for second row elements. J Chem Phys 77:3654
Frisch MJ, Pople JA, Binkley JS (1984) Self-consistent molecular orbital methods 25: supplementary functions for Gaussian basis sets. J Chem Phys 80:3265
Clark T, Chandrasekhar J, Spitznagel GW, Schleyer PR (1983) Efficient diffuse function-augmented basis sets for anion calculations. III. The 3-21+G set for first-row elements, Li-F. J Comput Chem 4:294
Schlegel HB, Frisch MJ (1995) Transformation between Cartesian and pure spherical harmonic Gaussians. Int J Quantum Chem 54:83
van Lenthe E, Snijders JG, Baerends EJ (1994) The zero-order regular approximation for relativistic effects: the effect of spin–orbit coupling in closed shell molecules. J Chem Phys 105:6505
Hehre WJ, Radom L, Schleyer PR, Pople JA (1986) Ab initio molecular orbital theory. Wiley, New York
Kendall RA, Dunning Jr TH, Harrison RJ (1992) Electron affinities of the first-row atoms revisited. Systematic basis sets and wave functions. J Chem Phys 96:6796
Foresman JB, Frisch A (1996) Exploring chemistry with electronic structure methods. Gaussian, Inc., Pittsburgh
Zhao Y, Truhlar DG (2008) The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor Chem Account 120:215
Glendening ED, Reed AE, Carpenter JE, Weinhold F NBO Version 3.1.
Domingo LR, Chamorro E, Pérez P (2008) Understanding the reactivity of captodative ethylenes in polar cycloaddition reactions. A theoretical study. J Organomet Chem 73:4615
Parr RG, Szentpaly L, Liu S (1999) Electrophilicity index. J Am Chem Soc 121:1922
Parr RG, Pearson RG (1983) Absolute hardness: companion parameter to absolute electronegativity. J Am Chem Soc 105:7512
Parr RG, Yang W (1989) Density functional theory of atoms and molecules. Oxford University Press, New York
Mizuhata Y, Sasamori T, Tokitoh N (2009) Stable heavier carbene analogues. Chem Rev 109:3479
Pettit WA, Wilson JW (1977) Substituent effects on a sigmatropic reaction. Rearrangement of some 3-substituted 1,1-diphenylindenes. J Am Chem Soc 99:6372
Bourissou D, Guerret O, Gabbaï FP, Bertrand G (2000) Stable Carbenes. Chem Rev 100:39
Rekken BD, Brown TM, Fettinger JC, Lips F, Tuononen HM, Herber RH, Power PP (2013) Dispersion forces and counterintuitive steric effects in main group molecules: heavier group 14 (Si-Pb) dichalcogenolate carbene analogues with sub-90° interligand bond angles. J Am Chem Soc 135:10134
Akbari A, Golzadeh B, Arshadi S, Kassaee MZ (2015) A quest for stable 2, 5-bis (halobora) cyclopentenylidene and its Si, Ge, Sn and Pb analogs at theoretical levels. RSC Adv 5:43319
Kassaee MZ, Arshadi S, Acedy M, Vessally E, Ghambarian M (2005) Singlet–triplet energy separations in divalent five-membered cyclic conjugated C5H3X, C4H3SiX, C4H3GeX, C4H3SnX, and C4H3PbX (X= H, F, Cl, and Br). J Organomet Chem 690:3427
Mekky ABH, Elhaes HG, El-Okr MM, Ibrahim MA (2015) Molecular electrostatic potential analysis of nano-scale fullerene (C60) crystals and some specific derivatives: DFT approach. J Nanomater Mol Nanotechnol 4:2
Grev RS, Schaefer HF, Gaspar PP (1991) In search of triplet silylenes. J Am Chem Soc 113:5638
Govindarajan M, Karabacak M, Suvitha A, Periandy S (2012) FT-IR, FT-Raman, ab initio, HF and DFT studies, NBO, HOMO-LUMO and electronic structure calculations on 4-chloro-3-nitrotoluene. Spectrochim Acta A Mol Biomol Spectrosc 89:137
Ruiz-Espinoza A, Ramos E, Salcedo R (2013) Theoretical description of benzene–fullerene and its organometallic derivative. Comput Theor Chem 1016:36
Dheivamalar S, Sugi L, Ambigai K (2016) Density functional theory study of exohedral carbon atoms effect on electrophilicity of nicotine: comparative analysis. Comput Chem 4:17
Dheivamalar S, Sugi L (2015) Density functional theory (DFT) investigations on doped fullerene with heteroatom substitution. Spectrochim Acta A Mol Biomol Spectrosc 151:687
Funding
This research is financially supported by the Technical and Vocational University of Tehran, Dr. Shariaty College, Tehran, and North Tehran Branch, Islamic Azad University, Tehran, Iran. The corresponding author and all the co-authors are aware of and approve of the submission (including the approval from the authorization or institution.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
The calculated XYZ Cartesian coordinates and shapes of selected frontier molecular orbitals for scrutinized plumbylenes (16 pages). (DOCX 3141 kb)
Rights and permissions
About this article
Cite this article
Koohi, M., Bastami, H. A quest for stable 2,2,9,9-tetrahaloplumbacyclonona-3,5,7-trienylidenes at density functional theory. Struct Chem 31, 877–898 (2020). https://doi.org/10.1007/s11224-019-01457-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-019-01457-z