Abstract
Vulvar squamous cell carcinoma can be divided by human papillomaviruses (HPV) status into two distinct clinicopathological and molecular entities. New agents targeting the tumor surface expression of programmed cell death-1/programmed cell death-ligand-1 are becoming a therapeutic option in an increasing number of carcinomas. We evaluate CD274 (PD-L1), CDKN2A (p16), tumor protein p53 (TP53), and epidermal growth factor receptor (EGFR) immunoexpression in primary tumors, recurrences and lymph node metastases and its correlations with prognosis and HPV status. We report 93 cases of vulvar squamous cell carcinoma diagnosed between 2002 and 2016 with the description of their clinicopathological features and prognosis data. Immunohistochemistry for CD274, CDKN2A, TP53, and EGFR was performed on tissue microarrays collecting from primary tumor, recurrences and lymph node metastasis. Kaplan–Meier estimator and multivariable Cox regression analysis controlling for FIGO stage and age were used. Patients who underwent surgery had a superior overall survival (HR = 0.51, 95% CI = 0.26–0.99 p = 0.04). Lymph node metastasis size ≥5 mm was associated with an inferior overall survival (HR = 1.88, 95% CI = 1.22–2.92 p = 0.004). CDKN2A expression was correlated with an inferior rate of recurrent disease (p = 0.02). In high-risk HPV DNA+ vulvar squamous cell carcinomas patients with CDKN2A− carcinomas showed a significantly worse overall survival than women with CDKN2A+ tumors (56% vs.100%, p = 0.003). TP53 expression was associated with an increased rate of recurrent disease (p = 0.0005). CD274 expression was associated with lymph node metastasis (p = 0.04). In 16 patients the CD274, CDKN2A, TP53, and EGFR expression changed between primary tumors, recurrences and lymph node metastases during tumor progression. In conclusion, a significant percentage of vulvar squamous cell carcinoma has a heterogeneous biomarker expression during tumor progression. We highlight the importance of some of these markers to be used as prognostic biomarkers. This data brings new light to future treatment using targeted therapy to EGFR or CD274 to include retesting such biomarkers in recurrence and lymph nodes metastases.
Similar content being viewed by others
Introduction
Vulvar squamous cell carcinoma accounts for 4% of cancers in the female reproductive organs and 0.6% of all cancers in women [1]. About 40% of the vulvar squamous cell carcinoma are human papillomaviruses (HPV) related [2, 3]. According to the presence or absence of HPV, vulvar squamous cell carcinoma has been divided into two distinct biological entities with different tumorigenic pathways: HPV positive (HPV+) and HPV negative (HPV−) [4]. HPV+ vulvar squamous cell carcinomas occurs in younger women, frequently have a basaloid or warty morphology and are associated with vulvar intraepithelial neoplasia of usual type and diffuse expression of cell cycle protein cyclin-dependent kinase inhibitor 2A (CDKN2A) [5,6,7,8]. CDKN2A immunohistochemistry has been shown to be a good surrogate marker of HPV infection in the vulva and in other anatomical sites. However, it is not known whether vulvar squamous cell carcinomas with inconclusive features (HPV DNA PCR+/CDKN2A− and HPV DNA PCR−/CDKN2A+) represent HPV positive or negative tumors [7, 8]. HPV negative vulvar squamous cell carcinoma arises predominantly in older women, is frequently of the keratinizing subtype and is associated with vulvar intraepithelial neoplasia of differentiated type and lichen sclerosus [9,10,11]. HPV− vulvar squamous cell carcinoma and its precursor vulvar intraepithelial neoplasia of differentiated type frequently share the same TP53 mutational profile, as TP53 is the most mutated gene in HPV− vulvar squamous cell carcinoma [12, 13]. The TP53 immunostaining detection is usually due to cellular accumulation of abnormal TP53 protein [14, 15].
Although HPV+ and HPV− vulvar squamous cell carcinomas have different pathways of tumorigenesis, an overlap in clinical and morphological characteristics is recognized [9, 16]. Cervical, anal and oropharyngeal HPV+ carcinomas are reported to have a higher survival rate due to better response to chemotherapy and radiation therapy, but the prognostic significance of HPV status in vulvar squamous cell carcinoma is still controversial [16,17,18,19].
Vulvar squamous cell carcinoma has a poor prognosis with a 2-year overall survival of 49,8% and about 30–50% of the patients developing recurrences within two years of the initial treatment [13, 16, 20]. Therapeutic options for recurrent disease are currently limited.
Altered epidermal growth factor receptor (EGFR) expression and activity have been associated with different epithelial cancers such as lung, colon, head and neck, and pancreatic cancer [21, 22]. Previous studies reported EGFR protein overexpression in a subset of HPV− vulvar squamous cell carcinoma with worse prognosis [23, 24]. However, other studies failed to reproduce this association [25]. Given the lack of consistency among different authors, the presence of EGFR overexpression in vulvar squamous cell carcinoma also needs to be clarified.
Currently, new agents targeting tumor surface expression of programmed cell death-1/programmed cell death-ligand-1 (PD-1/PD-L1) have been successfully used in the treatment of multiple solid tumors including cervical carcinoma [26]. In cervical carcinoma. CD274 expression has been associated with tumor metastases, tumor progression, and poor prognosis [26, 27]. Increased CD274 expression has been reported in HPV+ cervical carcinomas [28]. In vulvar squamous cell carcinoma, the impact of CD274 expression in prognosis is uncertain and its relationship with HPV status remains to be determined [29, 30].
Our aim is to evaluate the expression of CDKN2A, TP53, EGFR, and CD274 in vulvar squamous cell carcinoma (primary tumor, recurrences and lymph node metastases) and correlate these data with clinicopathological features (namely prognosis) and HPV status.
Materials and methods
Study population
The study population included 93 patients diagnosed with invasive vulvar squamous cell carcinoma and treated at the Instituto Português de Oncologia de Lisboa, Francisco Gentil, for 14 years (2002–2016). Clinical data was obtained from the clinical files and included: age at diagnosis, lymph nodes metastases, tumor size, surgical margin status, FIGO stage [31], recurrences, adjuvant therapy, and clinical outcome. This study was approved by the ethical committee of Instituto Português de Oncologia de Lisboa, Francisco Gentil.
Histological evaluation
All slides from each case (primary tumor, lymph node metastases and recurrences) were reviewed, and the diagnosis confirmed by two pathologists (AF and SL) (accordingly to WHO 2014), who were blinded to HPV results. The pathological parameters evaluated were histological type, tumor size and thickness, koilocytotic-like change, invasive front, peritumoral lymphocytic infiltrate (light; moderate or heavy), lymphovascular invasion, perineural invasion, surgical margins, lymph node status, and lymph node metastasis size. Representative paraffin-embedded tissue blocks of each case (primary tumor, lymph node metastases and recurrences) were selected to build a tissue microarray from the archives of the pathology department at the Instituto Português de Oncologia de Lisboa, Francisco Gentil.
Tissue microarray construction
For each case (primary tumor and recurrences) three 1.5 mm core biopsies were taken from the tumor core, invading tumor edge and normal epithelial-tumor transition respectively. For each lymph node with metastases, two 1.5 mm core biopsies were taken from the metastasis.
Immunohistochemistry
Immunohistochemistry for CDKN2A, TP53, EGFR, and CD274 was performed on 4-μm-thick sections obtained from the tissue microarrays.
Slides were stained on the BenchMark ULTRA IHC/ISH Automatic staining platform (Ventana Medical Systems) with anti-human monoclonal CDKN2A antibody (clone E6H4, pre-diluted, for 28 min; pretreatment ULTRA CC1–36 min, catalog number 805–4713, Ventana Medical Systems); anti-human monoclonal TP53 antibody (clone D07, dilution, 1:150 for 12 min; pretreatment ULTRA CC1–36 min, catalog number 453M-96 Cell Marque), anti-human EGFR Kit (clone 2–18C9, catalog number K1494, Agilent Dako) and anti-human monoclonal PD-L1 antibody (clone 22C3, dilution, 1:40 for 28 min; pretreatment ULTRA CC1–48 min, catalog number M3653, Agilent Dako), with appropriate positive and negative controls samples. Antigen detection was performed using OptiView DAB IHC Detection Kit (Ventana Medical Systems) with diaminobenzidine as the chromogen to detect antigen expression. Tissue sections were counterstained with Mayer's hematoxylin.
Scoring was performed by two pathologists (AF and SL), who were blinded to the clinical parameters. Nuclear CDKN2A was evaluated in a four-tier category: absent (<1%), focal (<25%), multifocal (25–70%), or diffuse (>70%). It was considered to be positive, if >70% of tumor cells were stained with CDKN2A, accordingly to the threshold used for head and neck HPV-associated squamous cell carcinoma [32]. Confirmation of CDKN2A immunohistochemistry result was done in whole tumor sections in all HPV DNA+/CDKN2A− cases. Concomitantly, nuclear TP53 was evaluated in a four-tier category: absent (<1%), focal (<25%), multifocal (25–50%), or diffuse (>50%), only if >50% of tumor nuclei were stained with TP53 was considered positive, accordingly with a threshold used for vulvar squamous cell carcinoma by Dong et al. [25]. EGFR was scored based on the intensity of membranous staining in a four-tier category: 0 (staining not greater than negative control or when staining was observed in <10% of the cells), 1+ (light), 2+ (moderate) or 3+ (strong), only 3+ was considered as positive, according to the scoring system used in vulvar carcinoma by several authors [23, 24, 33]. Scoring CD274 was based on the degree of membranous staining with a continuous honeycomb pattern. The expression of CD274 was categorized in four-tier category: absent (<1%), weak (1–5%), moderate (5–50%), or strong (>50%) and CD274 was considered positive if >1% of tumor cells showed membranous staining. This scoring was adopted from the criteria used for non-small-cell lung cancer [34, 35]. The primary tumor CD274+/recurrence or lymph node metastasis CD274− cases, were reevaluated in whole tumor sections.
HPV detection and typing
The DNA extraction was performed on fresh tissue from the pretreatment biopsy or tumor specimen. HPV DNA detection and genotyping were done using the SPF10 PCR, DEIA, LIPA25 system (version 2), which allows the identification of 32 HPV genotypes.
Statistical analysis
The statistical analysis was performed by using Kaplan–Meier estimator, and multivariable Cox regression analysis controlling for FIGO stage and age were used. A p value of <0.05 was considered statistically significant.
Results
Clinicopathological characteristics
The clinicopathological characteristics from the 93 patients are summarized in Table 1. Median age was 74 years (range 26–93). FIGO stage was I in 47%, II in 10%, and III in 43% of the cases. Lymph node metastases were present in 38 cases (41%), and median lymph node metastasis size was 8.5 mm (range 0.4–39 mm). Most of the patients (85) were initially treated with surgery, and 45 received adjuvant therapy. Only seven patients were treated with radiotherapy and chemotherapy. Disease recurrence was observed in 46 cases (49%). Seventeen patients (18%) developed regional lymph node metastases and two (2%) developed distant metastases. Within a median follow-up of 3.2 years, 35 of the patients (38%) died of the disease. Of these 35 patients, 33 died of local disease progression or recurrence without distant metastases.
Tumors were histologically classified as HPV-associated subtypes in seven cases (8%): five basaloid carcinomas and two hybrid carcinomas with basaloid and warty features. 73 cases (78%) were classified as keratinizing and 13 (14%) as nonkeratinizing. Most of the tumors were classified as moderately differentiated (55%) and 6 (7%) were multifocal. Median tumor size was 30 mm (range 1–110 mm) and the median depth of invasion was 5 mm (range 0.4–35). The invasive front was infiltrative in 69 carcinomas (75%) and pushing in 23 tumors (25%) (Table 2). Lymphovascular invasion was found in 13 cases, and 15 cases had perineural invasion. Forty-three cases (46%) had moderate peritumoral lymphocytic infiltrate and 33 (35%) had a light peritumoral lymphocytic infiltrate. Associated vulvar intraepithelial neoplasia of usual type and vulvar intraepithelial neoplasia of differentiated type were identified respectively in 20 cases and 9 cases (Table 2). HPV infection was determined in all cases: 64 cases (69%) were HPV DNA− and 29 (31%) were HPV DNA+ (11 HPV DNA16 positive) (Table 3). Of the 29 HPV DNA+ vulvar squamous cell carcinoma, 21 cases (72%) were of the keratinizing subtype, four cases (14%) of nonkeratinizing subtype and four (14%) of the histologically HPV-associated subtypes (basaloid and hybrid).
Immunohistochemical characteristics
Tissue microarray included 84 primary tumors, 30 lymph node metastases, 33 first recurrences and 14 s recurrences. Some of the paraffin-embedded blocks of primary tumors were unavailable, which explains the discrepancy between the number of patients and samples analyzed to build the tissue microarrays. Some immunostainings are not reported in primary tumors, recurrences and lymph node metastases due to limited material in the tissue microarray (diagnostic biopsies). Results of the immunohistochemical study are presented in Tables 3, 4, and 5.
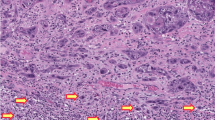
CDKN2A was positive in 12 of the 84 primary tumors (14%). Among the 72 cases that were CDKN2A−, the pattern of staining was absent in 63 cases (75%), focal in 5 cases (6%) and multifocal in 4 cases (5%). Nine of the 26 HPV DNA+ primary tumors were CDKN2A+. Eight of the 11 HPV16 DNA+ primary tumors were CDKN2A+ (Table 3). In 3 of the HPV DNA+/CDKN2A− cases, the HPV subtypes identified were HPV6 and HPV53. Two of the 30 lymph node metastases (7%) were CDKN2A+. Positive CDKN2A staining was detected in 4/29 of the first recurrences (14%) and 2/12 of the second recurrences (17%) (Table 4). In one case (HPV DNA−) where CDKN2A staining was negative in primary tumor, the recurrence was CDKN2A+ and, also TP53+ (Fig. 1). All the CDKN2A+ primary tumors maintained the CDKN2A staining pattern in recurrent disease.
a Primary tumor (invading tumor edge) hematoxylin and eosin (H&E); b first recurrence H&E; c H&E image of second recurrence; d negative CDKN2A in primary tumor; e positive CDKN2A in 1st recurrence; f positive CDKN2A in 2nd recurrence; g positive TP53 in primary tumor; h positive TP53 in 1st recurrence; i positive TP53 in 2st recurrence.
TP53 was positive in 28 of the 84 primary tumors (33%). Among the 56 cases that were TP53 negative, the pattern of staining was absent in 28 cases (33%), focal in 13 cases (15%), and multifocal in 15 cases (18%). The distribution of TP53 immunohistochemistry and HPV DNA status is presented in Tables 3 and 5. TP53 showed positive staining in 8 of the 29 lymph node metastases (28%), in 12 of the 29 first recurrences (41%), and 3 of 12 s recurrences (25%) (Table 4). In two cases where TP53 staining was negative in primary tumors, lymph node metastases were positive. In positive TP53 primary tumors, three of the recurrences and one of lymph node metastasis were TP53 negative (Fig. 2). Immunopositivity for both CDKN2A and TP53 was observed in one primary tumor.
EGFR was positive in 11 of the 84 primary tumors (13%), 4 of the 28 lymph node metastases (14%), and 3 of the 29 first recurrences (10%) (Table 4). It was negative in all of the second recurrences. Of the 73 negative primary tumors, 15 cases were scored as 0, 26 cases were scored as 1+ and 32 cases were scored as 2+. Among the EGFR positive primary tumors, five of the recurrences and one of the synchronous lymph node metastases were negative (Fig. 3). Two of the EGFR negative primary tumors became positive in recurrent disease. Only two cases of the 26 HPV DNA+ cases were EGFR positive (Table 3).
In the 83 cases of primary tumors, CD274 expression was absent (<1%) in 62 cases and present in 21 cases (weak, moderate, and strong expression in respectively 5, 4, and 12 cases). CD274 was positive in 9 of the 30 lymph node metastases (30%), 4 of the 33 first recurrences (12%) and 4 of the 14 s recurrences (29%) (Table 4). Five of the recurrences and 2 of the metachronous lymph node metastases of the 62 CD274 negative primary tumors became CD274 positive. In CD274 positive primary tumors, 4 of the recurrences (2 with strong CD274 expression) and 2 of the metachronous lymph node metastases lost CD274 expression (Fig. 4). Five cases of the HPV DNA+ group were CD274 positive (Table 3). CD274 was positive in 13 out of the 35 primary tumors with FIGO stage III (37%) and in 8 out of the 41 primary tumors with FIGO stage I (20%).
Statistical analysis
HPV DNA+ tumors were more likely to have koilocytotic change (p = 0.006). HPV DNA− tumors were associated with vulvar intraepithelial neoplasia of differentiated type (p = 0.03). There was no significant statistical difference in the remaining evaluated morphological parameters (Table 2).
No differences in overall survival were found between HPV DNA+ and HPV DNA− tumors. Patients who underwent surgery had a longer overall survival (HR = 0.51, 95% CI = 0.26–0.99 p = 0.032) (Fig. 5). The presence of lymph node metastases larger or equal to 5 mm was associated with an inferior OS (HR = 1.88, 95% CI = 1.22–2.92 p = 0.004) (Fig. 6).
Since the discrepancy between CDKN2A and HPV DNA status was significant (Table 3), we performed a logrank test to evaluate the prognostic value of CDKN2A immunostaining in the 18 patients with high-risk HPV DNA vulvar squamous cell carcinoma. HPV DNA+/CDKN2A− tumors showed worse 3-year overall survival than HPV DNA+/CDKN2A+ tumors (56% vs. 100%) in the HPV DNA+ vulvar squamous cell carcinoma group (p = 0.003). CDKN2A expression was associated with an inferior rate of recurrent disease (p = 0.02). TP53 expression was associated with a superior rate of recurrent disease (p = 0.0005). CD274 expression was positively associated with lymph node metastases (p = 0.04). CDKN2A expression was inversely associated with TP53 immunophenotype (p = 0.05). Expression of CDKN2A, TP53, EGFR, or CD274 was not associated with any other morphological characteristics or overall survival (Table 5). EGFR and CD274 expression were also not associated with HPV status or CDKN2A expression.
Discussion
In our series, only 31% of the vulvar squamous cell carcinomas were HPV DNA+, of which 12% were HPV DNA16+. These tumors were more likely to display koilocytotic-like change, but this was not a uniform finding. The weak correlation observed between morphologic features and HPV infection is related to significant interobserver variability in distinguishing true koilocytotic change from pseudokoilocytotic changes as already reported by other authors [7, 9, 36]. Regarding histological subtypes, the vast majority (71%) of the HPV DNA+ cases was of the keratinizing subtype, characteristic of the HPV− vulvar squamous cell carcinoma. All vulvar squamous cell carcinomas associated with vulvar intraepithelial neoplasia of differentiated type were HPV DNA− and predominantly (89%) of conventional keratinizing type, as shown by others [14]. However, in three cases (6%) of the HPV DNA− cases, the histological subtypes (basaloid and hybrid) were characteristic of HPV DNA+ vulvar squamous cell carcinoma. Our results support previous studies that histologic subtypes and morphologic features, although strongly associated with HPV infection are inadequate to differentiate between HPV+ and HPV− vulvar squamous cell carcinoma [7]. The relationship between HPV status and prognosis in vulvar squamous cell carcinoma has also conflicting results in the literature. In our series, no differences in overall survival between HPV DNA-positive and -negative tumors was observed, similar to what Alonso et al. reported [16]. In contrast, other studies have shown that the presence of HPV or CDKN2A overexpression in vulvar squamous cell carcinoma is associated with better survival, specifically in patients treated with radiotherapy [7, 19, 37].
In our series, patients who underwent surgery as primary treatment, had a superior OS compared with other treatments adjusted to the age and FIGO stage. Our results may be related to the extended time of the study (14 years), leading to a heterogeneous patient population concerning their treatment. Also, the low HPV prevalence that we have reported could explain a possible resistance of the tumors to radiotherapy and a better overall survival of the patients treated with surgery. Prognosis was also related to lymph node metastasis size, with patients with lymph node metastases larger or equal to 5 mm having an inferior overall survival controlled by stage and age.
About 90% of cervical carcinomas are HPV+ and CDKN2A immunostaining is used as a surrogate marker in this setting [38]. Moreover, in oropharynx carcinoma, CDKN2A is a routine tool used to classify into HPV+ and HPV− subgroups [39]. Intense and diffuse expression of CDKN2A reflects functional inactivation of RB induced by viral oncoprotein E7, which in turn releases E2F allowing the cell to enter in the S phase of cell cycle [40]. Previous large series of vulvar squamous cell carcinomas report a high sensitivity and specificity of CDKN2A immunohistochemical detection for HPV infection [9].
Interestingly, in our series, 17 cases (65%) of the HPV DNA+ primary tumors were CDKN2A negative (in 14 cases, CDKN2A staining was absent and 3 cases was focal or multifocal). The mismatch between CDKN2A overexpression and HPV DNA status was also reported by other groups [8, 41]. The confirmed absence of CDKN2A expression by immunohistochemistry can be related to tumor heterogeneity, the HPV type, and also due to further somatic mutations in the genome among the high-risk HPV-associated carcinomas [42]. In three of these cases, the detected HPV type was a low-risk type (HPV6 and HPV53). In this situation, CDKN2A staining is expected to be negative [7, 43]. In another of the cases, the HPV type was HPV66, a virus with limited evidence of carcinogenic effect in cervical cancer [8]. Although CDKN2A immunostaining was not informative of HPV DNA status, patients with CDKN2A− vulvar squamous cell carcinoma associated with high-risk HPV DNA+ showed a significantly worse overall survival than women with CDKN2A+ tumors (56% vs. 100%, p = 0.003). This association is similar to the recently reported results in cervical carcinoma [44]. Furthermore, we found that 3 (5%) of the HPV DNA− primary tumors expressed CDKN2A. These cases might represent a clearance of the HPV in tumors initially driven by HPV or represent false-negative results of HPV DNA detection has it was purposed by Nicolás et al. [38]. One CDKN2A− case in the primary tumor (HPV DNA−) became positive in recurrence, which may be explained by a gain of a mutation in RB gene leading to an RB protein inactivation, irrespective of HPV infection [25].
In vulvar squamous cell carcinoma HPV− cases, the direct inactivation of TP53 by missense and deletion mutations is frequent. TP53 staining is frequently detected in keratinizing squamous cell carcinoma HPV unrelated because of the cellular accumulation of the mutated abnormal protein [15]. No association was found between TP53 immunoexpression and HPV DNA. One case of vulvar squamous cell carcinoma of the keratinizing subtype (HPV DNA−) revealed the co-expression of TP53 and CDKN2A, suggesting that the CDKN2A overexpression may be seen in a minority of HPV DNA− vulvar squamous cell carcinoma. Although CDKN2A and TP53 expression are mostly mutually exclusive in vulvar squamous cell carcinoma, both in this cohort and in the literature, their rare co-expression has also been documented [25]. In positive TP53 primary tumor, three of the recurrences and one of the lymph node metastases were negative, suggesting that the loss of TP53 expression may be associated with a "de novo" mutation in TP5 or tumor heterogeneity. Also, three of these patients received adjuvant therapy with radiotherapy, which could induce the molecular alteration in the recurrence.
EGFR amplification is present in a subset of vulvar squamous cell carcinoma and represents a therapeutic target of tyrosine kinase inhibitors. Indeed, a case report of two patients with vulvar squamous cell carcinoma treated with erlotinib revealed promising results [45]. A previous observation showed that 31% of vulvar squamous cell carcinoma were EGFR positive, irrespective of HPV status [24]. In our series, we report a lower prevalence of EGFR immunoexpression. Only 11 cases (13%) of our 84 primary tumors were EGFR positive. Two of these cases were HPV DNA+ and all were CDKN2A−. This observation was previously reported in head and neck squamous cell carcinoma, where tumors with EGFR protein overexpression are more frequently HPV-independent. These findings suggest that EGFR activation may be involved in HPV-independent tumorigenesis.
Among the EGFR positive primary tumors, five of the recurrences and one of the synchronous lymph node metastases were negative. The finding might be valuable in the discussion of where and when to evaluate EGFR if it is going to be used as a therapeutic target. Though it may also be related to the tissue microarray limitation in evaluating the EGFR heterogeneity staining, as Melo Maia et al. described [46].
Immunotherapy with anti-PD-1/PD-L1 checkpoint inhibitors has been approved in a variety of tumor types, including cervical squamous cell carcinoma [47]. In the literature, one case of a recurrent vulvar squamous cell carcinoma successfully treated with pembrolizumab was described [48]. Different studies report that the overexpression of CD274 in vulvar squamous cell carcinoma is present in 31–71% of the cases, which may be related to the different scoring methods [49, 50]. The association between HPV status and CD274 is controversial, as some groups described that CD274 is unrelated to HPV status, and other groups showed CD274 expression was more frequent in vulvar squamous cell carcinoma HPV− [50, 51]. In our series, CD274 expression was positive in 25% of the primary vulvar squamous cell carcinoma, irrespective of HPV status. In the CD274 positive primary tumor group, four recurrences and two metachronous lymph node metastases lost CD274 expression. These results were reevaluated and confirmed in whole tumor sections, suggesting that it may occur an alteration in the tumoral microenvironment during the progression of the disease. All the patients in this subgroup were treated with adjuvant radiotherapy, which may contribute to this alteration.
Regarding these biomarkers and their use as prognosis markers, our data showed that the expression of CDKN2A was associated with HPV DNA+ vulvar squamous cell carcinoma. As described in previous studies, the CDKN2A expression correlated with an inferior rate of recurrent disease (p = 0.02), and positive TP53 expression was associated with a higher rate of recurrent disease (p = 0.0005) [7, 25, 37]. EGFR expression was not associated with clinicopathological characteristics. The overexpression of the CD274 has been reported as being independent of HPV status and associated with poor clinical outcomes in several immunogenic cancers, but in vulvar squamous cell carcinoma, the observations are controversial [52]. In our series, the CD274 expression was not related to HPV status but was found to be associated with the presence of lymph node metastasis, which has never been described in vulvar squamous cell carcinoma, indicating a poor clinical outcome. This observation was previously observed in the cervical squamous cell carcinoma [27]. The CD274 expression was more frequent in advanced stage, suggesting some clinical impact of the CD274 expression in tumor progression, corroborating a recent study [30]. These results suggest that patients probably will benefit more from checkpoint inhibitors, however further comprehensive analyses are warranted.
The limitations of this retrospective study include an extended period duration study with an inherent heterogeneity in patients’ treatment, such as surgical approach and radiotherapy doses. The immunohistochemical study in tissue microarray samples can also be considered a limitation. Intratumoral heterogeneity introduces problems in the evaluation of immunohistochemistry expression that are increased in tissue microarrays. For that reason, we tried to minimize this problem by sampling up to three cores for each case, and confirming, in tumor whole sections, part of our immunohistochemistry results.
In conclusion, our results suggest that CDKN2A− tumors in high-risk HPV DNA+ have a significantly worse prognosis in the vulvar squamous cell carcinoma. This result also points that the CDKN2A immunoexpression in vulvar squamous cell carcinoma is not only a surrogate marker of HPV DNA but has added prognostic value.
Our results also demonstrate that in 16 patients (20 immunostains), the expression of CDKN2A, TP53, EGFR, and CD274 was heterogeneous between primary tumors, recurrences and lymph node metastases, suggesting that biomarkers immunoexpression changes during the progression of the disease. Furthermore, these results suggest that if a targeted therapy to EGFR or CD274 is to be used, these biomarkers should be retested in recurrences and lymph node metastases. These differences in biomarker expression may be explained by dynamic alterations of the tumor microenvironment, which should be clarified on further studies.
References
Saraiya M, Watson M, Wu X, King JB, Chen VW, Smith JS, et al. Incidence of in situ and invasive vulvar cancer in the US, 1998–2003. Cancer. 2008;113:2865–72.
Van De Nieuwenhof HP, Van Kempen LCLT, De Hullu JA, Bekkers RLM, Bulten J, Melchers WJG, et al. The etiologic role of HPV in vulvar squamous cell carcinoma fine tuned. Cancer Epidemiol Biomark Prev. 2009;18:2061–7.
De Vuyst H, Clifford GM, Nascimento MC, Madeleine MM, Franceschi S. Prevalence and type distribution of human papillomavirus in carcinoma and intraepithelial neoplasia of the vulva, vagina and anus: a meta-analysis. Int J Cancer. 2009;124:1626–36.
Moody CA, Laimins LA. Human papillomavirus oncoproteins: pathways to transformation. Nat Rev Cancer. 2010;10:550–60.
Kurman RJ, Toki T, Schiffman MH. Basaloid and warty carcinomas of the vulva. Distinctive types of squamous cell carcinoma frequently associated with human papillomaviruses. Am J Surg Pathol. 1993;17:133–45.
Toki T, Kurman RJ, Park JS, Kessis T, Daniel RW, Shah KV. Probable nonpapillomavirus etiology of squamous cell carcinoma of the vulva in older women: a clinicopathologic study using in situ hybridization and polymerase chain reaction. Int J Gynecol Pathol. 1991;10:107–25.
Rakislova N, Clavero O, Alemany L, Saco A, Quirós B, Lloveras B, et al. “Histological characteristics of HPV-associated and -independent squamous cell carcinomas of the vulva: a study of 1,594 cases”. Int J Cancer. 2017;141:2517–27.
Sanjose S, Alemany L, Ordi J, Tous S, Alejo M, Sanjose S De, et al. Worldwide human papillomavirus genotype attribution in over 2000 cases of intraepithelial and invasive lesions of the vulva. Eur J Cancer. 2013;49:3450–61.
Santos M, Landolfi S, Olivella A, Lloveras B, Klaustermeier J, Campo E, et al. p16 overexpression identifies HPV-positive vulvar. Am J Surg Pathol. 2006;30:1347–56.
Singh N, Leen SL, Han G, Faruqi A, Kokka F, Rosenthal A, et al. Expanding the morphologic spectrum of differentiated VIN (dVIN) through detailed mapping of cases with p53 Loss. Am J Surg Pathol. 2015;39:52–60.
Watkins J. Human papillomavirus– independent squamous lesions of the vulva. Surg Pathol. 2019;12:249–61.
Pinto AP, Miron A, Yassin Y, Monte N, Woo TYC, Mehra KK, et al. Differentiated vulvar intraepithelial neoplasia contains Tp53 mutations and is genetically linked to vulvar squamous cell carcinoma. Mod Pathol. 2010;23:404–12.
Han M, Shin S, Park H, Kim MS, Lee SH, Jung SH, et al. Mutational signatures and chromosome alteration profiles of squamous cell carcinomas of the vulva. Exp Mol Med. 2018;50:e442.
Yang B, Hart WR. Vulvar intraepithelial neoplasia of the simplex (differentiated) type: a clinicopathologic study including analysis of HPV and p53 expression. Am J Surg Pathol. 2000;24:429–41.
Santos M, Montagut C, Mellado B, García Á, Ramón S, Cardesa A, et al. Immunohistochemical staining for p16 and p53 in premalignant and malignant epithelial lesions of the vulva. Int J Gynecol Pathol. 2004;23:206–14.
Alonso I, Fusté V, Del Pino M, Castillo P, Torné A, Fusté P, et al. Does human papillomavirus infection imply a different prognosis in vulvar squamous cell carcinoma? Gynecol Oncol. 2011;122:509–14.
McAlpine JN, Leung SCY, Cheng A, Miller D, Talhouk A, Gilks CB, et al. Human papillomavirus (HPV)-independent vulvar squamous cell carcinoma has a worse prognosis than HPV-associated disease: a retrospective cohort study. Histopathology. 2017;71:238–46.
Lassen P, Eriksen JG, Hamilton-Dutoit S, Tramm T, Alsner J, Overgaard J. Effect of HPV-associated p16 INK4A expression on response to radiotherapy and survival in squamous cell carcinoma of the head and neck. J Clin Oncol. 2009;27:1992–8.
Lee L, Howitt B, Catalano P, Tanaka C, Murphy R, Cimbak N, et al. Prognostic importance of human papillomavirus (HPV) and p16 positivity in squamous cell carcinoma of the vulva treated with radiotherapy. Gynecol Oncol. 2016;142:293–8.
Horne ZD, Dohopolski MJ, Pradhan D, Bhargava R, Edwards RP, Kelley JL, et al. Gynecologic Oncology Human papillomavirus infection mediates response and outcome of vulvar squamous cell carcinomas treated with radiation therapy. Gynecol Oncol. 2018;151:96–101.
Ciardiello F, Tortora G. EGFR antagonists in cancer treatment. N Engl J Med. 2008;358:1160–74.
Hynes NE, Lane HA. ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer. 2005;5:341–54.
Brustmann H. Epidermal growth factor receptor is involved in the development of an invasive phenotype in vulvar squamous lesions, but is not related to MIB-1 immunoreactivity. Int J Gynecol Pathol. 2007;26:481–9.
Growdon WB, Boisvert SL, Akhavanfard S, Oliva E, Dias-Santagata DC, Kojiro S, et al. Decreased survival in EGFR gene amplified vulvar carcinoma. Gynecol Oncol. 2008;111:289–97.
Dong F, Kojiro S, Borger DR, Growdon WB, Oliva E. Squamous cell carcinoma of the vulva: a subclassification of 97 cases by clinicopathologic, immunohistochemical, and molecular features (p16, p53, and EGFR). Am J Surg Pathol. 2015;39:1045–53.
Liu Y, Wu L, Tong R, Yang F, Yin L, Li M, et al. PD-1/PD-L1 inhibitors in cervical cancer. Front Pharmacol. 2019;1:10.
Yang W, Lu Y-P, Yang Y-Z, Kang J-R, Jin Y-D, Wang H-W. Expressions of programmed death (PD)-1 and PD-1 ligand (PD-L1) in cervical intraepithelial neoplasia and cervical squamous cell carcinomas are of prognostic value and associated with human papillomavirus status. J Obstet Gynaecol Res. 2017;43:1602–12.
Mezache L, Paniccia B, Nyinawabera A, Nuovo GJ. Enhanced expression of PD L1 in cervical intraepithelial neoplasia and cervical cancers. Mod Pathol. 2015;28:1594–602.
Howitt BE, Sun HH, Roemer MGM, Kelley A, Chapuy B, Aviki E, et al. Genetic basis for PD-L1 expression in squamous cell carcinomas of the cervix and vulva. JAMA Oncol. 2016;2:518–22.
Thangarajah F, Morgenstern B, Pahmeyer C, Schiffmann LM, Puppe J, Mallmann P, et al. Clinical impact of PD-L1 and PD-1 expression in squamous cell cancer of the vulva. J Cancer Res Clin Oncol. 2019;145:1651–60.
Committee F. FIGO staging for carcinoma of the vulva, cervix, and corpus uteri. Int J Gynaecol Obstet. 2014;125:97–8.
Singhi AD, Westra WH. Comparison of human papillomavirus in situ hybridization and p16 immunohistochemistry in the detection of human papillomavirus-associated head and neck cancer based on a prospective clinical experience. Cancer. 2010;116:2166–73.
Berchuck A, Rodriguez G, Kamel A, Soper JT, Clarke-Pearson DL, Bast RC. Expression of epidermal growth factor receptor and HER-2/neu in normal and neoplastic cervix, vulva, and vagina. Obstet Gynecol. 1990;76:381–7.
Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389:255–65.
Fehrenbacher L, Spira A, Ballinger M, Kowanetz M, Vansteenkiste J, Mazieres J, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet. 2016;387:1837–46.
McCluggage W, Walsh M, Thornton C, Hamilton P, Date A, Caughley LMBH. Inter- and intra-observer variation in the histopathological reporting of cervical squamous intraepithelial lesions using a modified Bethesda grading system. Br J Obs Gynaecol. 1998;105:206–10.
Yap ML, Allo G, Cuartero J, Pintilie M, Kamel-reid S, Murphy J, et al. Prognostic significance of human papilloma virus and p16 expression in patients with vulvar squamous cell carcinoma who received radiotherapy. Clin Oncol (R Coll Radiol). 2018;30:254–61.
Nicolás I, Marimon L, Barnadas E, Saco A, Rodríguez-Carunchio L, Fusté P, et al. HPV-negative tumors of the uterine cervix. Mod Pathol. 2018;32:1189–96.
Klussmann JP, Gültekin E, Weissenborn SJ, Wieland U, Dries V, Dienes HP, et al. Expression of p16 protein identifies a distinct entity of tonsillar carcinomas associated with human papillomavirus. Am J Pathol. 2003;162:747–53.
Fonmarty D, Cherrière S, Fleury H, Eimer S, Majoufre-lefebvre C, Castetbon V. Study of the concordance between p16 immunohistochemistry and HPV-PCR genotyping for the viral diagnosis of oropharyngeal squamous cell carcinoma. Eur Ann Otorhinolaryngol Head Neck Dis. 2015;132:135–9.
Sznurkowski JJ, Żawrocki A, Biernat W. The overexpression of p16 is not a surrogate marker for high-risk human papilloma virus genotypes and predicts clinical outcomes for vulvar cancer. BMC Cancer. 2016;16:465.
Banister CE, Liu C, Pirisi L, Creek KE, Buckhaults PJ. Identification and characterization of HPV-independent cervical cancers. Oncotarget. 2017;8:13375–86.
Liu Y, Alqatari M, Sultan K, Ye F, Gao D, Sigel K, et al. Using p16 immunohistochemistry to classify morphologic cervical intraepithelial neoplasia 2: correlation of ambiguous staining patterns with HPV subtypes and clinical outcome. Hum Pathol. 2017;66:144–51.
Nicolás I, Saco A, Barnadas E, Marimon L, Rakislova N, Fusté P, et al. Prognostic implications of genotyping and p16 immunostaining in HPV-positive tumors of the uterine cervix. Mod Pathol. 2019. https://doi.org/10.1038/s41379-019-0360-3. [Epub ahead of print].
Clancy A, Spaans J, Weberpals J. The forgotten woman’s cancer: vulvar squamous cell carcinoma (VSCC) and a targeted approach to therapy. Ann Oncol. 2016;27:1696–705.
De Melo Maia B, Fontes AM, Lavorato-Rocha AM, Rodrigues ISA, De Brot L, Baiocchi G, et al. EGFR expression in vulvar cancer: clinical implications and tumor heterogeneity. Hum Pathol. 2014;45:917–25.
Chinn Z, Stoler MH, Mills AM. PD-L1 and IDO expression in cervical and vulvar invasive and intraepithelial squamous neoplasias: implications for combination immunotherapy. Histopathology. 2019;74:256–68.
Shields LBE, Gordinier ME. Pembrolizumab in recurrent squamous cell carcinoma of the vulva: case report and review of the literature. Gynecol Obstet Invest. 2019;84:94–8.
Choschzick M, Gut A, Fink D. PD-L1 receptor expression in vulvar carcinomas is HPV-independent. Virchows Arch. 2018;473:513–6.
Sznurkowski JJ, Żawrocki A, Sznurkowska K, Pęksa R, Biernat W. PD-L1 expression on immune cells is a favorable prognostic factor for vulvar squamous cell carcinoma patients. Oncotarget. 2017;8:89903–12.
Hecking T, Thiesler T, Schiller C, Lunkenheimer J-M, Ayub TH, Rohr A, et al. Tumoral PD-L1 expression defines a subgroup of poor-prognosis vulvar carcinomas with non-viral etiology. Oncotarget. 2017;8:92890–903.
Wang X, Teng F, Kong L, Yu J. PD-L1 expression in human cancers and its association with clinical outcomes. Onco Targets Ther. 2016;9:5023–39.
Acknowledgements
Part of this work was presented at the United States and Canadian Academy of Pathology (USCAP) 108th Annual Meeting, March 16–21, 2019 at Gaylord National Resort & Convention Center, National Harbor, Maryland, United States of America. We thank the excellent work of Dr Joana Ferreira reviewing the manuscript. We thank Cristina Azedo for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lérias, S., Esteves, S., Silva, F. et al. CD274 (PD-L1), CDKN2A (p16), TP53, and EGFR immunohistochemical profile in primary, recurrent and metastatic vulvar cancer. Mod Pathol 33, 893–904 (2020). https://doi.org/10.1038/s41379-019-0429-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41379-019-0429-z
This article is cited by
-
An integrated model for prognosis in vulvar squamous cell carcinoma
BMC Cancer (2023)