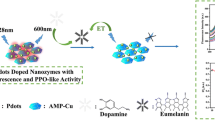
Abstract
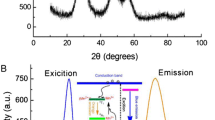
Laccases play a vital role in some physiological processes, for example in morphogenesis, carbon cycle, and defense against parasitism. So, designing a high-sensitivity accurate method is essential for researchers. In this study, a simple fluorescence method based on the function of carbon nitride (g-C3N4) by dopamine is synthesized. For the design of this sensor, carbon nitride (g-C3N4) is initially synthesis by using a simple method, which is carried out by heating melamine at 550 °C for 3 h and modifying it with dopamine by a linker such as glutaraldehyde. However, the g-C3N4–Dopa produced by this method, with an excitation wavelength of 330 nm, has a fluorescence emission at 466 nm. When laccase and g-C3N4–Dopa were mixed, dopamine with redox property was oxidized to dopaquinone; this causes the phenomenon of photoinduced electron transfer (PET) process between g-C3N4 and the dopaquinone. Hence, fluorescence quenching occurs due to this phenomenon. As a result of these discussions, a sensor for the laccase activity was designed based on the fluorescence quenching degree, supporting a linear range of 0.0–400.0 U L−1 with the detection limit of 2.0 U L−1. Using this sensor, the activity of the laccase enzyme in the human serum samples is measured.
Graphic abstract
Dopamine-functionalized carbon nitride was prepared and utilized for the highly sensitive detection of laccases activity.








Similar content being viewed by others
Change history
17 December 2020
The original article can be found online.
References
Pomerantz SH (1966) The tyrosine hydroxylase activity of mammalian tyrosinase. J Biol Chem 241(1):161–168
Singh G, Capalash N, Goel R, Sharma P (2007) A pH-stable laccase from alkali-tolerant γ-proteobacterium JB: purification, characterization and indigo carmine degradation. Enzyme Microb Technol 41(6–7):794–799
Sharma P, Goel R, Capalash N (2007) Bacterial laccases. World J Microbiol Biotechnol 23(6):823–834
Mogharabi M, Faramarzi MA (2014) Laccase and laccase-mediated systems in the synthesis of organic compounds. Adv Synth Catal 356(5):897–927
Xu F (2005) Applications of oxidoreductases: recent progress. Ind Biotechnol 1(1):38–50
Brijwani K, Rigdon A, Vadlani PV (2010) Fungal laccases: production, function, and applications in food processing. Enzyme Res 2010:149748
Baldrian P (2006) Fungal laccases—occurrence and properties. FEMS Microbiol Rev 30(2):215–242
Eichlerová I, Šnajdr J, Baldrian P (2012) Laccase activity in soils: considerations for the measurement of enzyme activity. Chemosphere 88(10):1154–1160
Martins LO, Soares CM, Pereira MM, Teixeira M, Costa T, Jones GH, Henriques AO (2002) Molecular and biochemical characterization of a highly stable bacterial laccase that occurs as a structural component of the Bacillus subtilis endospore coat. J Biol Chem 277(21):18849–18859
Slominski A, Tobin DJ, Shibahara S, Wortsman J (2004) Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol Rev 84(4):1155–1228
Esterbauer H, Schwarzl E, Hayn M (1977) A rapid assay for catechol oxidase and laccase using 2-nitro-5-thiobenzoic acid. Anal Biochem 77(2):486–494
Miller R, Kuglin J, Gallagher S, Flurkey W (1997) A spectrophotometric assay for laccase using o-tolidine. J Food Biochem 21(1):445–459
Shin T, Murao S, Matsumura E (1987) A chromogenic oxidative coupling reaction of laccase: applications for laccase and angiotensin I converting enzyme assay. Anal Biochem 166(2):380–388
Farnet A, Ferre E, Gil G, Gastaldi S (2010) A new substrate to measure laccase activities in complex environments: application to litters. Soil Biol Biochem 42(6):1001–1005
Volkova N, Ibrahim V, Hatti-Kaul R (2012) Laccase catalysed oxidation of syringic acid: calorimetric determination of kinetic parameters. Enzyme Microb Technol 50(4–5):233–237
Pashangeh K, Hormozi-Nezhad MR, Akhond M, Absalan G (2018) Laccase activity assay using surface plasmon resonance band of gold nanoparticles formed by dopamine. Plasmonics 13(4):1409–1415
Liu J-X, Zhou W-J, Gong J-L, Tang L, Zhang Y, Yu H-Y, Wang B, Xu X-M, Zeng G-M (2008) An electrochemical sensor for detection of laccase activities from Penicillium simplicissimum in compost based on carbon nanotubes modified glassy carbon electrode. Bioresour Technol 99(18):8748–8751
Huang H, Cai R, Du Y, Lin Z, Ye Zeng (1995) Micelle enhanced spectrofluorimetric assay for laccase activity by a flow-injection stopped-flow technique. Anal Chim Acta 318(1):63–69
Wang T, Xiang Y, Liu X, Chen W, Hu Y (2017) A novel fluorimetric method for laccase activities measurement using Amplex Red as substrate. Talanta 162:143–150
Rong M, Lin L, Song X, Zhao T, Zhong Y, Yan J, Wang Y, Chen X (2014) A label-free fluorescence sensing approach for selective and sensitive detection of 2,4,6-trinitrophenol (TNP) in aqueous solution using graphitic carbon nitride nanosheets. Anal Chem 87(2):1288–1296
Zhang P, Liu H, Ma S, Men S, Li Q, Yang X, Wang H, Zhang A (2016) A label-free ultrasensitive fluorescence detection of viable Salmonella enteritidis using enzyme-induced cascade two-stage toehold strand-displacement-driven assembly of G-quadruplex DNA. Biosens Bioelectron 80:538–542
Liu L, Liu J-W, Duan L-Y, Luo F-Y, Wang Y-M, Yu R-Q, Jiang J-H (2018) Graphitic carbon nitride nanosheets-based turn-on fluorescent biosensor for highly sensitive, label-free detection of adenylate kinase activity. Sens Actuators B Chem 267:231–236
Chai L, Zhou J, Feng H, Tang C, Huang Y, Qian Z (2015) Functionalized carbon quantum dots with dopamine for tyrosinase activity monitoring and inhibitor screening: in vitro and intracellular investigation. ACS Appl Mater Interfaces 7(42):23564–23574
Zhang H (2015) Ultrathin two-dimensional nanomaterials. ACS Nano 9(10):9451–9469
Lee EZ, Lee SU, Heo N-S, Stucky GD, Jun Y-S, Hong WH (2012) A fluorescent sensor for selective detection of cyanide using mesoporous graphitic carbon (IV) nitride. Chem Commun 48(33):3942–3944
Wang Y, Li Z, Weber TJ, Hu D, Lin C-T, Li J, Lin Y (2013) In situ live cell sensing of multiple nucleotides exploiting DNA/RNA aptamers and graphene oxide nanosheets. Anal Chem 85(14):6775–6782
Zhang L, Zhang Q, Lu X, Li J (2007) Direct electrochemistry and electrocatalysis based on film of horseradish peroxidase intercalated into layered titanate nano-sheets. Biosens Bioelectron 23(1):102–106
Zhang L, Zhang Q, Li J (2007) Layered titanate nanosheets intercalated with myoglobin for direct electrochemistry. Adv Funct Mater 17(12):1958–1965
Shiravand G, Badiei A, Ziarani GM (2017) Carboxyl-rich g-C3N4 nanoparticles: synthesis, characterization and their application for selective fluorescence sensing of Hg2+ and Fe3+ in aqueous media. Sens Actuators B Chem 242:244–252
Xie H, Dong J, Duan J, Hou J, Ai S, Li X (2019) Magnetic nanoparticles-based immunoassay for aflatoxin B1 using porous g-C3N4 nanosheets as fluorescence probes. Sens Actuators B Chem 278:147–152
Xu M, Yang G, Bi H, Xu J, Feng L, Yang D, Sun Q, Gai S, He F, Dai Y (2019) Combination of CuS and g-C3N4 QDs on upconversion nanoparticles for targeted photothermal and photodynamic cancer therapy. Chem Eng J 360:866–878
Zhan T, Tan Z, Wang X, Hou W (2018) Hemoglobin immobilized in g-C3N4 nanoparticle decorated 3D graphene-LDH network: direct electrochemistry and electrocatalysis to trichloroacetic acid. Sens Actuators B Chem 255:149–158
Wang L, Zhou G, Tian Y, Yan L, Deng M, Yang B, Kang Z, Sun H (2019) Hydroxyl decorated g-C3N4 nanoparticles with narrowed bandgap for high efficient photocatalyst design. Appl Catal B 244:262–271
Zhao X, Pan D, Chen X, Li R, Jiang T, Wang W, Li G, Leung DY (2019) g-C3N4 photoanode for photoelectrocatalytic synergistic pollutant degradation and hydrogen evolution. Appl Surf Sci 467:658–665
Karami C, Taher MA (2019) A novel enzyme-less amperometric sensor for hydrogen peroxide based on nickel molybdate nanoparticles. J Electroanal Chem 847:113219
Karami C, Taher MA (2019) A catechol biosensor based on immobilizing laccase to Fe3O4@ Au core-shell nanoparticles. Int J Biol Macromol 129:84–90
Arkan E, Karami C, Rafipur R (2019) Immobilization of tyrosinase on Fe3O4@Au core–shell nanoparticles as bio-probe for detection of dopamine, phenol and catechol. JBIC J Biol Inorg Chem 24(7): 961–969
Ahmadi E, Eyvani MR, Riahifar V, Momeneh H, Karami C (2019) Amperometric determination of nevirapine by GCE modified with c-MWCNTs and synthesized 11-mercaptoundecanoyl hydrazinecarbothioamide coated silver nanoparticles. Microchem J 146:1218–1226
Karami C, Mehr SY, Deymehkar E, Taher MA (2018) Naked eye detection of Cr3+ and CO2+ ions by gold nanoparticle modified with azomethine. Plasmonics 13(2):537–544
Duan J, Zhang Y, Yin Y, Li H, Wang J, Zhu L (2018) A novel “on-off-on” fluorescent sensor for 6-thioguanine and Hg2+ based on g-C3N4 nanosheets. Sens Actuators B Chem 257:504–510
Moorthy M, Kannan B, Madheswaran B, Rangappan R (2016) Tethering of Cu(II) Schiff base metal complex on mesoporous material MCM-41: catalyst for Ullmann-type coupling reactions. J Porous Mater 23(4):977–986
Wang C-C, Li J-R, Lv X-L, Zhang Y-Q, Guo G (2014) Photocatalytic organic pollutants degradation in metal–organic frameworks. Energy Environ Sci 7(9):2831–2867
Guan E, Xie M, Shi Y-e, Zhang X, Zhan J (2016) Dimolecular interaction between graphitic carbon nitride nanosheets and phenols: a mechanism study. Carbon 102:462–469
Ma TY, Tang Y, Dai S, Qiao SZ (2014) Proton-functionalized two-dimensional graphitic carbon nitride nanosheet: an excellent metal-/label-free biosensing platform. Small 10(12):2382–2389
Cheng N, Jiang P, Liu Q, Tian J, Asiri AM, Sun X (2014) Graphitic carbon nitride nanosheets: one-step, high-yield synthesis and application for Cu2+ detection. Analyst 139(20):5065–5068
Cuadrado MU, Pérez-Juan PM, de Castro MDL, Gómez-Nieto MA (2005) A fully automated method for in real time determination of laccase activity in wines. Anal Chim Acta 553(1–2):99–104
Jiang C, Ling S, Wang P, Liang A, Chen B, Wen G, Jiang Z (2011) A new and sensitive catalytic resonance scattering spectral assay for the detection of laccase using guaiacol as substrate. Luminescence 26(6):500–505
Liang A, Wang P, Jiang Z (2011) A new and sensitive catalytic resonance scattering spectral assay for the detection of laccase activity using H2O2-I−-TDMAC system. Chin J Chem 29(4):787–792
Acknowledgements
The authors gratefully acknowledge the Medical Cellular and Molecular Research Center of Golestan University of Medical Sciences (Grant Number: 111255) for the financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Arabi, M.S., Karami, C., Taher, M.A. et al. Fluorescence detection of laccases activity by the photoinduced electron transfer (PET) process. J Biol Inorg Chem 25, 151–159 (2020). https://doi.org/10.1007/s00775-019-01748-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00775-019-01748-0