Abstract
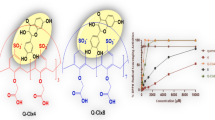
In this work, mixed ligand complexes of Co(II) Ni(II) and Cu(II) were synthesized using quercetin and diimine (1,10-phenanthroline or 2,2′-bipyiridine) ligands. The obtained Ni(II) and Co(II) complexes are new and the Cu(II) complexes are synthesized by different method from the literature. The characterization of complexes was performed by elemental analysis, thermogravimetric analysis, ESI–MS, UV–visible and infrared spectral analyses, magnetic susceptibility and molar conductivity measurements. It was found that quercetin, diimine and metal(II) ion form 1:1:1 complexes. Resulting data supported octahedral geometry for Ni(II) and Co(II) complexes and square pyramidal geometry for Cu(II) complexes. The proposed compositions are [Co(queH-1)Cl(phen)(H2O)]∙2H2O (1, queH = quercetin, phen = 1,10-phenanthroline), [Ni(queH-1)Cl(phen)(H2O)]∙2H2O (2), [Cu(queH-1)Cl(phen)]∙2.5H2O (3) and [Cu(queH-1)Cl(bpy)]∙2H2O (4, bpy = 2,2′-bipyiridine). Antioxidant capacity and total phenolic content of complexes measured by Folin–Ciocalteu and ABTS methods. Anti-cancer effect of these compounds were tested against different cancer cells (A549, PC-3, HeLa and MCF-7). Apoptosis identified by the fluorescence imaging, caspase cleaved cytokeratin-18 and flow cytometry analysis (annexin V, caspase 3/7, mitochondria membrane potential and oxidative stress). As a result, Cu(II) complexes are more effective than the other compounds and Complex 3 is a promising anti-cancer compound against breast cancer MCF-7 and MDA-MB-231 cells (IC50 values are 2.4 and 5.4 µM for 48 h, respectively). Flow cytometry analysis exhibited that Complex 3 caused apoptosis in MCF-7 cells. These results support that Complex 3 has anticancer activity and can be a potential anticancer agent especially in breast cancer.










Similar content being viewed by others
References
Celiz G, Daz M, Audisio MC (2011) Antibacterial activity of naringin derivatives against pathogenic strains. J Appl Microbiol 111(3):731–738
Kandaswami C, Lee LT, Lee PP, Hwang JJ, Ke FC, Huang YT, Lee MT (2005) The antitumor activities of flavonoids. Vivo 19(5):895–909
Prochazkova D, Bousova I, Wilhelmova N (2011) Antioxidant and prooxidant properties of flavonoids. Fitoterapia 82(4):513–523
Wleklik M, Luczak M, Panasiak W, Kobus M, Lammer-Zarawska E (1988) Structural basis for antiviral activity of flavonoids-naturally occurring compounds. Acta Virol 32(6):522–525
Jayaraman J, Jesudoss VAS, Menon VP, Namasivayam N (2012) Anti-inflammatory role of naringenin in rats with ethanol induced liver injury. Toxicol Mech Methods 22(7):568–576
Bansal P, Paul P, Mudgal J, Nayak PG, Pannakal ST, Priyadarsini KI, Unnikrishnan MK (2012) Antidiabetic, antihyperlipidemic and antioxidant effects of the flavonoid rich fraction of Pilea microphylla (L.) in high fat diet/streptozotocin-induced diabetes in mice. Exp Toxicol Pathol 64(6):651–658
Grazul M, Budzisz E (2009) Biological activity of metal ions complexes of chromones, coumarins and flavones. Coordin Chem Rev 253(21–22):2588–2598
De Souza RFV, De Giovani WF (2004) Antioxidant properties of complexes of flavonoids with metal ions. Redox Rep 9(2):97–104
Lin X, Lin C-H, Zhao T, Zuo D, Ye Z, Liu L, Lin M-T (2017) Quercetin protects against heat stroke-induced myocardial injury in male rats: antioxidative and antiinflammatory mechanisms. Chem Biol Interact 265:47–54
Mandal SM, Dias RO, Franco OL (2017) Phenolic compounds in antimicrobial therapy. J Med Food 20(10):1031–1038
Yao H, Xu W, Shi X, Zhang Z (2011) Dietary flavonoids as cancer prevention agents. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev 29(1):1–31
Ohnishi E, Bannai H (1993) Quercetin potentiates TNF-induced antiviral activity. Antiviral Res 22(4):327–331
Sandhir R, Mehrotra A (1832) Quercetin supplementation is effective in improving mitochondrial dysfunctions induced by 3-nitropropionic acid: implications in Huntington’s disease. Biochim Biophys Acta. Mol Basis Dis 3:421–430
Dehghan G, Khoshkam Z (2012) Tin(II)–quercetin complex: synthesis, spectral characterisation and antioxidant activity. Food Chem 131(2):422–426
Porkodi J, Raman N (2018) Synthesis, characterization and biological screening studies of mixed ligand complexes using flavonoids as precursors. Appl Organomet Chem 32(2):e4030
Dolatabadi JEN (2011) Molecular aspects on the interaction of quercetin and its metal complexes with DNA. Int J Biol Macromol 48(2):227–233
Zhou J, Wang L, Wang J, Tang N (2001) Antioxidative and anti-tumour activities of solid quercetin metal(II) complexes. Transit Metal Chem 26(1–2):57–63
Zhou J, Wang L, Wang J, Tang N (2001) Synthesis, characterization, antioxidative and antitumor activities of solid quercetin rare earth(III) complexes. J Inorg Biochem 83(1):41–48
Bukhari SB, Memon S, Mahroof-Tahir M, Bhanger MI (2008) Synthesis, characterization and investigation of antioxidant activity of cobalt–quercetin complex. J Mol Struct 892(1–3):39–46
Bukhari SB, Memon S, Mahroof-Tahir M, Bhanger MI (2009) Synthesis, characterization and antioxidant activity copper–quercetin complex. Spectrochim Acta A 71(5):1901–1906
Ravichandran R, Rajendran M, Devapiriam D (2014) Antioxidant study of quercetin and their metal complex and determination of stability constant by spectrophotometry method. Food Chem 146:472–478
Chen W, Sun S, Cao W, Liang Y, Song J (2009) Antioxidant property of quercetin–Cr(III) complex: the role of Cr(III) ion. J Mol Struct 918(1–3):194–197
Kang J, Zhuo L, Lu X, Liu H, Zhang M, Wu H (2004) Electrochemical investigation on interaction between DNA with quercetin and Eu–Qu3 complex. J Inorg Biochem 98(1):79–86
Abou-El-Sherbini KhS, Hassanien MM (2004) Synthesis of controlled-pore silica glas functionalized with quercetin and its application for the separation and preconcentration of Mn(II), Co(II), Ni(II), Cu(II), and Zn(II). Sep Sci Technol 39(5):1177–1201
De Souza RFV, De Giovani WF (2005) Synthesis, spectral and electrochemical properties of Al(III) and Zn(II) complexes with flavonoids. Spectrochim Acta A 61(9):1985–1990
Andelescu AA, Cretu C, Sasca V, Marinescu S, Cseh L, Costisor O, Szerb EI (2018) New heteroleptic Zn(II) and Cu(II) complexes with quercetine and N^N ligands. Polyhedron 147:120–125
Vimalraj S, Rajalakshmi S, Preeth DR, Kumar SV, Deepak T, Gopinath V, Murugan K, Chatterjee S (2018) Mixed-ligand copper(II) complex of quercetin regulate osteogenesis and angiogenesis. Mater Sci Eng C Mater Biol Appl 83(1):187–194
Alper P, Erkisa M, Mutlu Gençkal H, Şahin S, Ulukaya E, Ari F (2019) Synthesis, characterization, anticancer and antioxidant activity of new nickel(II) and copper(II) flavonoid complexes. J Mol Struct 1196:783–792
Sarria ALF, Vilela AFL, Frugeri BM, Fernandes JB, Carlos RM, Fernandes da Silva DGMF, Cass QB, Cardoso CL (2016) Copper (II) and zinc (II) complexes with flavanone derivatives: identification of potential cholinesterase inhibitors by on–flow assays. J Inorg Biochem 164:141–149
Balogh-Hergovich E, Kaizer J, Pap J, Speier G, Huttner G, Zsolnai L (2002) Copper-mediated oxygenolysis of flavonols via endoperoxide and dioxetan intermediates; synthesis and oxygenation of [CuII(Phen)2(Fla)]ClO4 and [CuII(L)(Fla)2] [FlaH = Flavonol; L = 1,10-Phenanthroline (Phen), 2,2′-Bipyridine (Bpy), N,N,N′,N′,-Tetramethylethylenediamine (TMEDA)] complexes. E. Eur J Inorg Chem 9:2287–2295
Oliveira RMM, de Souza Daniel JF, Carlos RM (2013) Synthesis, spectroscopic characterization and biological activity of cis–[Ru(hesperidin)(1,10-phenanthroline)2](PF6) complex. J Mol Struct 1031:269–274
Wang Q, Huang M, Huang Y, Zhang J-S, Zhou G-F, Zeng R-Q, Yang X-B (2014) Synthesis, characterization, DNA interaction, and antitumor activities of mixed-ligand metal complexes of kaempferol and 1,10-phenanthroline/2,2′-bipyridine. Med Chem Res 23(5):2659–2666
Filho JCC, Sarria ALF, Becceneri AB, Fuzer AM, Batalhão JR, da Silva CMP, Carlos RM, Vieira PC, Fernandes JB, Cominetti MR (2014) Copper (II) and 2,2′-bipyridine complexation improves chemopreventive effects of naringenin against breast tumor cells. PLoS One 9(9):e107058
Xiao B, Wang H, Zhao X (2014) Selective recognition of luteolin and quercetin based on the specific interaction of ortho-dihydroxy substituents with a zinc(II) complex. Anal Methods 6:2894–2899
Tamayo LV, Gouvea LR, Sousa AC, Albuquerque RM, Teixeira SF, Azevedo RA, Louro SRW, Ferreira AK, Beraldo H (2016) Copper(II) complexes with naringenin and hesperetin: cytotoxic activity against A 549 human lung adenocarcinoma cells and investigation on the mode of action. Biometals 29(1):39–52
Sahin S, Aybastıer O, Işık E (2013) Optimisation of ultrasonic-assisted extraction of antioxidant compounds from Artemisia absinthium using response surface methodology. Food Chem 141(2):1361–1368
Ali I, Wani WA, Saleem K (2013) Empirical formulae to molecular structures of metal complexes by molar conductance. Synth React Inorg M 43(9):1162–1170
Geary WJ (1971) The use of conductivity measurements in organic solvents for the characterisation of coordination compounds. Coord Chem Rev 7(1):81–122
El-Behery M, El-Twigry H (2007) Synthesis, magnetic, spectral, and antimicrobial studies of Cu(II), Ni(II) Co(II), Fe(III), and UO2(II) complexes of a new Schiff base hydrazone derived from 7–chloro–4–hydrazinoquinoline. Spectrochim Acta A Mol Biomol Spectrosc 66(1):28–36
Ibrahim MM, Ramadan AEM, Shaban SY, Mersal GAM, El-Shazly SA, Al-Juaid S (2017) Syntheses, characterization and antioxidant activity studies of mixed ligand copper(II) complexes of 2,2′-bipyridine and glycine: the X-ray crystal structure of [Cu(BPy)(Gly)]ClO4. J Mol Struct 1134:319–329
Inci D, Aydın R, Yılmaz D, Mutlu Gençkal H, Vatan Ö, Çinkılıç N, Zorlu Y (2015) New water-soluble copper (II) complexes including 4,7-dimethyl-1,10-phenanthroline and l-tyrosine: synthesis, characterization, DNA interactions and cytotoxicities. Spectrochim Acta A Part B(4):761–770
Kanakis CD, Tarantilis PA, Polissiou MG, Diamantoglou S, Tajmir-Riahi HA (2007) An overview of DNA and RNA bindings to antioxidant flavonoids. Cell Biochem Biophys 49(1):29–36
Musualik M, Kuzmicz R, Pawlowski TS, Litwinienko G (2009) Acidity of hydroxyl groups: an overlooked influence on antiradical properties of flavonoids. J Org Chem 74(7):2699–2709
Naso L, Valcarcel M, Villacé P, Roura-Ferrer M, Salado C, Ferrer EG, Williams PAM (2014) Specific antitumor activities of natural and oxovanadium(IV) complexed flavonoids in human breast cancer cells. New J Chem 38:2414–2421
Zhai G, Zhu W, Duan Y, Qu W, Yan Z (2012) Synthesis, characterization and antitumor activity of the germanium-quercetin complex. Main Group Met Chem 35(3–4):103–109
Tabassum S, Zaki M, Afzal M, Arjmand F (2013) New modulated design and synthesis of quercetin-CuII/ZnII-Sn2 IV scaffold as anticancer agents: in vitro DNA binding profile, DNA cleavage pathway and Topo-I activity. Dalton Trans 42(27):10029–10041
Naso L, Martínez VR, Lezama L, Salado C, Valcarcel M, Ferrer EG, Williams PAM (2016) Antioxidant, anticancer activities and mechanistic studies of the flavone glycoside diosmin and its oxidovanadium(IV) complex. Interactions with bovine serum albumin. Bioorg Med Chem 24(18):4108–4119
Fazary AE, Ju Y-H, Al-Shihri AS, Bani-Fwaz MZ, Alfaifi MY, Alshehri MA, Saleh KA, Elbehairi SEI, Fawy KF, Abd-Rabboh HSM (2017) Platinum and vanadate bioactive complexes of glycoside naringin and phenolates. Open Chem 15(1):189–199
Ragazzon PA, Bradshaw T, Matthews C, Iley J, Missailidis S (2009) The characterisation of flavone-DNA isoform interactions as a basis for anticancer drug development. Anticancer Res 29(6):2273–2283
Křikavová R, Vančo J, Trávníček Z, Hutyra J, Dvořák Z (2016) Design and characterization of highly in vitro antitumor active ternary copper (II) complexes containing 2′-hydroxychalcone ligands. J Inorg Biochem 163:8–17
Spoerlein C, Mahal K, Schmidt H, Schobert R (2013) Effects of chrysin, apigenin, genistein and their homoleptic copper(II) complexes on the growth and metastatic potential of cancer cells. J Inorg Biochem 127:107–115
Chien S-Y, Wu Y-C, Chung J-G, Yang J-S, Lu H-F, Tsou M-F, Wood WG, Kuo S-J, Chen D-R (2009) Quercetin-induced apoptosis acts through mitochondrial and caspase-3-dependent pathways in human breast cancer MDA-MB-231 cells. Hum Exp Toxicol 28(8):493–503
Kumar S, Pandey A (2013) Chemistry and biological activities of flavonoids: an overview. Sci World J (ID 162750), pp 1–16
Ong CS, Tran E, Nguyen TTT, Ong CK, Lee SK, Lee JJ, Ng CP, Leong C, Huynh H (2004) Quercetin-induced growth inhibition and cell death in nasopharyngeal carcinoma cells are associated with increase in Bad and hypophosphorylated retinoblastoma expressions. Oncol Rep 11(3):727–733
Khan I, Paul S, Jakhar R, Bhardwaj M, Han J, Kang SC (2016) Novel quercetin derivative TEF induces ER stress and mitochondria-mediated apoptosis in human colon cancer HCT-116 cells. Biomed Pharmacother 84:789–799
Liao H, Bao X, Zhu J, Qu J, Sun Y, Ma X, Wang E, Guo X, Kang Q, Zhen Y (2015) O-Alkylated derivatives of quercetin induce apoptosis of MCF-7 cells via a caspase-independent mitochondrial pathway. Chem Biol Interact 242:91–98
Bao XR, Liao H, Qu J, Sun Y, Guo X, Wang EX, Zhen YH (2016) Synthesis, characterization and cytotoxicity of alkylated quercetin derivatives. Iran J Pharm Res 15(3):329–335
Tan M, Zhu J, Pan Y, Chen Z, Liang H, Liu H, Wang H (2009) Synthesis, cytotoxic activity, and DNA binding properties of copper (II) complexes with hesperetin, naringenin, and apigenin. Bioinorg Chem Appl 2009:347872
Selvaraj S, Krishnaswamy S, Devashya V, Sethuraman S, Krishnan UM (2012) Membrane fluidization & eryptotic properties of hesperidin-copper complex. RSC Adv 2(29):11138–11146
Etcheverry SB, Ferrer EG, Naso L, Rivadeneira J, Salinas V, Williams PA (2008) Antioxidant effects of the VO(IV) hesperidin complex and its role in cancer chemoprevention. J Biol Inorg Chem 13:435–447
Wu Q, Needs PW, Lu Y, Kroon PA, Ren D, Yang X (2018) Different antitumor effects of quercetin, quercetin-3′-sulfate and quercetin-3-glucuronide in human breast cancer MCF-7 cells. Food Funct 9(3):1736–1746
Liu H, Zhou M (2017) Antitumor effect of Quercetin on Y79 retinoblastoma cells via activation of JNK and p38 MAPK pathways. BMC Complement Altern Med 17:531–538
Zhang JY, Yi T, Liu J, Zhao ZZ, Chen HB (2013) Quercetin induces apoptosis via the mitochondrial pathway in KB and KBv200 cells. J Agric Food Chem 61(9):2188–2195
Tan J, Wang B, Zhu L (2009) DNA binding and oxidative DNA damage induced by a quercetin copper (II) complex: potential mechanism of its antitumor properties. J Biol Inorg Chem 14(5):727–739
Gokbulut AA, Apohan E, Baran Y (2013) Resveratrol and quercetin-induced apoptosis of human 232B4 chronic lymphocytic leukemia cells by activation of caspase-3 and cell cycle arrest. Hematology 18(3):144–150
Niu G, Yin S, Xie S, Li Y, Nie D, Ma L, Wang X, Wu Y (2011) Quercetin induces apoptosis by activating caspase-3 and regulating Bcl-2 and cyclooxygenase-2 pathways in human HL-60 cells. Acta Biochim Biophys Sin 43(1):30–37
Granado-Serrano AB, Martín MA, Bravo L, Goya L, Ramos S (2006) Quercetin induces apoptosis via caspase activation, regulation of Bcl-2, and inhibition of PI-3-kinase/Akt and ERK pathways in a human hepatoma cell line (HepG2). J Nutr 136(11):2715–2721
Chou CC, Yang JS, Lu HF, Ip SW, Lo C, Wu CC, Lin JP, Tang NY, Chung JG, Chou MJ, Teng YH, Chen DR (2010) Quercetin-mediated cell cycle arrest and apoptosis involving activation of a caspase cascade through the mitochondrial pathway in human breast cancer MCF-7 cells. Arch Pharm Res 33(8):1181–1191
Lakroun Z, Kebieche M, Lahouel A, Zama D, Desor F, Soulimani R (2015) Oxidative stress and brain mitochondria swelling induced by endosulfan and protective role of quercetin in rat. Environ Sci Pollut Res Int 22(10):7776–7781
Psotova J, Chlopcikova S, Grambal F, Simanek V, Ulrichova J (2002) Influence of silymarin and its flavonolignans on doxorubicin-iron induced lipid peroxidation in rat heart microsomes and mitochondria in comparison with quercetin. Phytother Res 16(Suppl. 1):S63–S67
Priyadarsini RV, Murugan RS, Maitreyi S, Ramalingam K, Karunagaran D, Nagini S (2010) The flavonoid quercetin induces cell cycle arrest and mitochondria-mediated apoptosis in human cervical cancer (HeLa) cells through p53 induction and NF-κB inhibition. Eur J Pharmacol 649(1–3):84–91
Yoshino S, Hara A, Sakakibara H, Kawabata K, Tokumura A, Ishisaka A, Kawai Y, Terao J (2011) Effect of quercetin and glucuronide metabolites on the monoamine oxidase-A reaction in mouse brain mitochondria. Nutrition 27(7–8):847–852
Zhang XM, Chen J, Xia YG, Xu Q (2005) Apoptosis of murine melanoma B16-BL6 cells induced by quercetin targeting mitochondria, inhibiting expression of PKC-α and translocating PKC-δ. Cancer Chemother Pharmacol 55(3):251–262
De Oliveira MR, Nabavi SM, Braidy N, Setzer WN, Ahmed T, Nabavi SF (2016) Quercetin and the mitochondria: a mechanistic view. Biotechnol Adv 34(5):532–549
Jakubowicz-Gil J, Langner E, Bądziul D, Wertel I, Rzeski W (2014) Quercetin and sorafenib as a novel and effective couple in programmed cell death induction in human gliomas. Neurotox Res 26(1):64–77
Galati G, O’brien PJ (2004) Potential toxicity of flavonoids and other dietary phenolics: significance for their chemopreventive and anticancer properties. Free Radic Biol Med 37(3):287–303
Acknowledgements
We thank G. Done for their technical assistance and the Research Fund of Bursa Uludag University for the financial support given to the research project (Project Number OUAP (F)–2014/27).
Funding
This work was supported by grants from the Research Fund of Bursa Uludag University for the financial support given to the research Project (Project Number OUAP (F)–2014/27).
Author information
Authors and Affiliations
Contributions
FA, HMG, and EU contributed to the study design, FA, HMG, ME and PA performed experiments, FA and HMG, performed the analysis of the data, SS setup und performed phenolic contents and antioxidant capacities of complexes, FA and HMG, contributed to the writing of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflicts of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mutlu Gençkal, H., Erkisa, M., Alper, P. et al. Mixed ligand complexes of Co(II), Ni(II) and Cu(II) with quercetin and diimine ligands: synthesis, characterization, anti-cancer and anti-oxidant activity. J Biol Inorg Chem 25, 161–177 (2020). https://doi.org/10.1007/s00775-019-01749-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00775-019-01749-z