Abstract
Aims/hypothesis
Both arachidonic acid (AA, 20:4 n-6) and docosahexaenoic acid (DHA,22:6 n-3), long-chain polyunsaturated fatty acids (LCPUFA), are involved in fetal development and, based on their percentage compositions, appear to be specifically accumulated in fetal circulation in a proposed phenomenon known as biomagnification. Discrepancies exist in the literature concerning the effect of gestational diabetes mellitus (GDM) on circulating fatty acids. Our objective was to analyse individual fatty acid concentrations in a large cohort of maternal and cord paired serum samples from pregnant women with and without GDM.
Methods
Overnight fasted maternal and cord blood paired samples from 84 women with GDM and well controlled blood glucose levels and 90 healthy pregnant women (controls) were drawn at term. Individual fatty acids within total serum lipids were analysed by gas chromatography and expressed both as concentrations of fatty acid (mmol/l) and as a percentage of total fatty acids.
Results
In the serum of overnight fasted pregnant women with GDM, the concentrations of most fatty acids were lower than in control women, except for AA and DHA, which remained the same. The concentrations of most fatty acids in cord serum were also lower in the GDM group than in the control group, except for α-linolenic acid (ALA,18:3 n-3), which was higher in the GDM group. In both groups, the concentrations of all fatty acids were lower in cord serum than in maternal serum. In GDM participants only, a positive and significant correlation between cord and maternal serum concentration of AA and DHA was observed.
Conclusions/interpretation
The expression of fatty acids in molar concentrations reveals that GDM decreases the concentration of most fatty acids in both maternal and cord serum. There is a high fetal dependence on maternal AA and DHA, but our findings do not support the existence of a fetal biomagnification of those two LCPUFA.
Similar content being viewed by others

Introduction
During intrauterine development, fatty acids play a critical role in fetal energy metabolism and cell differentiation, as well as in the regulation of inflammatory and immune responses. Additionally, fatty acids play complex roles in the control of transcription factors that regulate gene expression. The third trimester of pregnancy is characterised by a substantial increase in fetal growth and adipose tissue mass and, consequently, a high demand for maternal nutrients by the fetus [1]. During this period the supply of fatty acids, especially long-chain polyunsaturated fatty acids (LCPUFA), is essential for the appropriate development of the nervous system and retina [2,3,4]. Decreased fetal content of LCPUFA, arachidonic acid (AA, 20:4 n-6) or docosahexaenoic acid (DHA, 22:6 n-3) in particular, has been associated with intrauterine growth restriction [5], shorter duration of gestation [6] or delayed postnatal development [7] among other factors. These reports underline the importance of LCPUFA in perinatal growth.
Pregnancies affected by gestational diabetes mellitus (GDM) constitute an unfavourable condition in which fetal growth patterns are altered [8, 9]. Additionally, there is emerging evidence that points to GDM contributing to neurodevelopmental disorders [10] and cognitive impairment in offspring [11, 12], but normalisation of maternal glucose levels does not guarantee improvements in pregnancy outcomes, indicating that factors other than glucose actively contribute to fetal growth [13]. We have previously shown that, in pregnant women with GDM and good glycaemic control, maternal lipids, especially triacylglycerols (TAG) and NEFA, are strong predictors of both fetal lipids and fetal growth [14, 15]. However, the relationship between fetal fatty acid profiles at the end of pregnancy and GDM outcomes is difficult to determine, basically because there is no consensus on the effect of diabetes on the amount of circulating fatty acids. Some studies have reported that levels of circulating AA and DHA in women with GDM are lower than in control participants [16,17,18], but other studies found the opposite [19,20,21] or report that fatty acid profiles do not differ between pregnant women with and without GDM [22, 23]. These discrepancies are also found in studies on fetal fatty acids [18, 19, 24], where results are scarcer.
It is possible that the manner in which individual fatty acid data are expressed may have affected the findings, since most studies expressed their results as a percentage of total fatty acids. In contrast to common practice with other blood lipids, very few studies have reported the concentrations of different fatty acids in whole serum (or plasma) in women with GDM [20, 25] and, as far as we know, none of the studies in fetal serum. Based on the importance of certain fatty acids in fetal development, the aim of this study was to analyse molar concentration values of individual fatty acids in a large number of maternal and cord paired serum samples from pregnancies in women with and without GDM.
Methods
Study design and participants
The study included 174 women and their neonates (90 control participants and 84 participants with GDM). Women in both control and GDM groups gave birth at the Department of Obstetrics of the Vivantes Medical Center in Berlin, where the population was recruited at two different time periods as described previously [26, 27]. Women with GDM were participating in a study designed to examine metabolic variables in pregnancies complicated with GDM [26]. Offspring birthweight and height were obtained shortly after delivery; neonatal skinfold thickness at the flank was measured within 48 h to calculate fat mass by a formula derived from Catalano et al [28]. In all cases, inclusion criteria were: delivery after 34 weeks of gestation, absence of identified fetal anomalies, and singleton pregnancy. Gestational age was calculated from the last menstrual period and confirmed by an ultrasound examination performed before 20 weeks of gestation. Diagnosis of GDM was established by a 75 g OGTT at 26 weeks of gestation, interpreted according to the Carpenter and Coustan criteria [29]. The women self-monitored blood glucose. Additional insulin therapy was given based principally on maternal glucose levels or on fetal growth, as described previously [26]. Women had been advised to follow a diet of about 45–50% carbohydrate with high fibre content, 20% fat and 30% protein. All participating mothers gave informed written consent after having received verbal and written information on the study. The study protocol was approved by the local ethics committee.
Blood samples
Maternal blood samples were taken from a radial vein after an overnight fast, either on the morning of admission for surgery in case of primary Caesarean sections (C-sections) or at the last visit to the obstetric clinic, no longer than 1 week before delivery. Cord blood samples were taken from one of the umbilical arteries from a segment of the cord, immediately after delivery. Maternal and cord blood samples were centrifuged (1500 g at 4°C for 25 min) and aliquots of serum were immediately stored at −80°C until analysis. None of the samples used in the study showed haemolysis.
Analytical determinations
Serum glucose, cholesterol, TAG (Menarini Diagnostic, Florence, Italy), glycerol (Sigma, St Louis, MO, USA) and NEFA (Wako Chemicals, Germany) were determined enzymatically using commercial kits. Serum insulin concentration was measured using a sandwich ELISA kit according to the manufacturers’ instructions (Mercodia, Uppsala, Sweden). Profiles of total fatty acids were processed as previously described [30] and comprise NEFA, TAG, phospholipid and cholesteryl ester profiles. Serum lipids were extracted in chloroform:methanol (2:1) containing 0.005% (wt/vol.) butylated hydroxytoluene and an internal standard of nonadecanoic acid (19:1). The final lipid extracts were evaporated to dryness under vacuum, resuspended in toluene and subjected to methanolysis for 2.5 h at 80°C in methanol:toluene (4:1) containing acetyl chloride and methyl-heptadecanoate (17:0) as a reference standard. The fatty acid methyl esters were separated and quantified on a Perkin Elmer gas chromatograph (Autosystem; Norwalk, CT, USA), with a flame ionisation detector and a 30 m × 0.25 mm Omegawax (Sigma) capillary column. Nitrogen was used as carrier gas and the fatty acid methyl esters were compared with purified standards (Sigma). Quantification of the fatty acids in maternal and cord serum was performed as a function of the corresponding peak areas compared with those of the internal standard. Individual fatty acids were also expressed as a percentage of total fatty acids in the sample (g/100 g fatty acids). Specifically, the individual fatty acids measured were: palmitic acid (PA, 16:0), palmitoleic acid (POA, 16:1), stearic acid (SA, 18:0), oleic acid (OA, 18:1 n-9), linoleic acid (LA, 18:2 n-6), α-linolenic acid (ALA, 18:3 n-3), γ-linolenic acid (GLA, 18:3 n-6), dihomo-γ-linolenic acid (DGLA, 20:3 n-6), AA, eicosapentaenoic acid (EPA, 20:5 n-3) and DHA.
Statistics
Results are expressed as means ± SEM. Statistical difference among groups was determined by ANOVA, after adjustment for maternal pre-pregnancy BMI, gestational age at time of blood collection, sex of neonate, maternal insulin treatment and type of delivery (i.e. vaginal delivery or C-section) (the last three as binary variables) as possible confounding factors; when differences were statistically significant, multiple comparisons were performed using the Tukey post hoc test. Because their distributions were skewed, levels of insulin and fatty acids were log10-transformed before statistical comparison. All statistical analysis was performed using a computer software package (Statgraphics Centurion XVII, version 17.1.12; Statistical Graphics Corporation, The Plains, VA, USA).
Results
Table 1 summarises the characteristics of the cohorts. Pre-gestational BMI was slightly higher in women assigned to the GDM group, although at delivery they had the same BMI as the control participants. In addition, there were no differences observed in the duration of gestation between control and GDM participants. Moreover, glucose fasting during the OGTT at 26 weeks of gestation was higher in women with GDM than in control participants, although only 34% of GDM women needed insulin treatment. At the end of gestation, all pregnant women had well controlled blood glucose, with serum glucose concentration comparable in both groups. Maternal plasma insulin close to delivery was higher in the GDM group than the control group and umbilical cord glucose and insulin were also higher in the GDM than the control group. Maternal and cord serum glucose, insulin, TAG, cholesterol, glycerol and NEFA concentrations in GDM did not differ statistically between those of mothers that had been treated with insulin and those just with diet, without insulin (electronic supplementary material [ESM] Table 1). The percentage of C-sections was lower in the GDM group than the control group, and therefore this point was taken into account as a covariable in the statistical analysis. There was the same percentage of female and male newborns in both groups, and no statistical differences were found in neonatal characteristics such as birthweight, neonatal fat mass or the body fat percentage (neonatal fat mass to birthweight ratio) between control and GDM groups. Only placental weight was significantly lower in GDM than in the control group, although birthweight/placental weight ratio was statistically similar in participants with and without GDM (Table 1).
The fatty acid profile in total lipids from maternal and cord serum of pregnant women in the control and GDM groups, expressed as a percentage of total fatty acids, is shown in Fig. 1a–c. In both groups, PA, OA and LA were, proportionately, the most abundant fatty acids in maternal serum, and represented around 75% of total fatty acids present in the maternal circulation. Far below those percentages, AA and DHA were the most abundant LCPUFA, comprising around 5.5% and 2.5%, respectively, of all fatty acids circulating in maternal serum. In the cord serum of both groups PA and OA were also the most abundant fatty acids present, although the percentages of those fatty acids were significantly lower than in the mothers. The proportions of the polyunsaturated fatty acid (PUFA) precursors of the n-6 and n-3 LCPUFA series, LA and ALA, respectively, were lower in cord than in maternal serum. However, the percentage of the other LCPUFA components of the n-6 (GLA, DGLA) and n-3 (EPA, DHA) series were significantly higher in cord than in maternal serum, with the only exception being EPA, which showed a similar percentage in both maternal and cord serum (Fig. 1c). Compared with the control group, maternal serum from women in the GDM group showed a statistically lower proportion of PA and its metabolic products, such as POA and OA (Fig. 1a). In addition, the proportion of EPA was lower in maternal serum of the GDM group. By contrast, the maternal serum percentages of LA, AA and DHA were significantly higher in the GDM than the control group but there was no difference for GLA or DGLA. Similarly, the cord serum of women with GDM had significantly lower percentages of PA, SA, DGLA and EPA than control samples, while the percentage of OA, LA, GLA and ALA were higher than observed in control cord serum. However, no differences were found in the proportions of POA, AA or DHA in cord serum in the GDM group compared with the control group.
Fatty acid composition in maternal and cord serum of control (n=90) and GDM (n=84) samples. Data expressed as percentage (g/100 g fatty acids) of (a) SFA and MUFA, (b) n-6 and (c) n-3 fatty acids, and as concentration (mmol/l) of (d) SFA and MUFA, (e) n-6 and (f) n-3 fatty acids. Maternal and cord serum fatty acids were adjusted for maternal pre-pregnancy BMI, gestational age at time of blood collection, maternal insulin treatment and delivery mode of neonate; additionally, cord serum fatty acids were adjusted for sex of neonate. All fatty acids were log10-transformed for statistical comparisons. **p<0.01, ***p<0.001 for cord vs maternal serum in the same group; †p<0.05; ††p<0.01; †††p<0.001 for control vs GDM groups, as shown. All values are mean ± SEM; individual data points are shown
When the total lipid fatty acid profile in serum samples was expressed in molar concentrations (Fig. 1d–f), rather than relative percentages, a completely different and more interesting pattern emerged. On the one hand, in the GDM group the maternal serum concentrations of all individual fatty acids were significantly lower than those observed in the control group, with only two exceptions: AA and DHA, for which the concentrations were very similar in the two groups. Interestingly, in both the GDM and control groups, the concentrations of all individual fatty acids were significantly lower in cord than in maternal serum. Furthermore, cord serum from the GDM compared with the control group showed a significant reduction in the serum concentrations of all fatty acids with the only exception being ALA, which was higher in the GDM than the control group (0.005 ± 0.001 vs 0.007 ± 0.001 mmol/l in the control and GDM group, respectively), although quantitatively this fatty acid had a lower concentration than any other fatty acid present in cord circulation. Fatty acid concentrations were similar in samples obtained from vaginal and C-section (ESM Figs 1–3), and thus differences in fatty acid concentrations were maintained when we analysed only control and GDM samples from C-section delivery, although the low number in the GDM group reduced the statistical value (ESM Table 2). When maternal and cord serum fatty acid concentrations values were analysed in those pregnant women with GDM being treated with insulin or with just diet (without insulin), no differences in any fatty acid concentration was found (ESM Table 3). Similarly, no significant correlations between insulin, HOMA or glucose/insulin ratios in maternal or cord serum vs their respective individual fatty acid concentrations were found in women with GDM treated with insulin or just with diet (data not shown).
In both groups, the cord/maternal ratios of serum fatty acid percentages (g/100 g of total fatty acids) were <1 for PA, OA and the two essential fatty acids, LA and ALA, indicating that those fatty acids were present at a higher percentage in maternal serum than in fetal serum (Fig. 2a–c). The rest of the fatty acids showed a cord/maternal ratio >1, and this was especially so for the LCPUFA, AA and DHA (Fig. 2b, c), suggesting their preferential accumulation on the fetal side of the placenta. However, when cord/maternal serum ratios of individual fatty acids were expressed as actual serum concentrations (Fig. 2d–f), no value was found >1, indicating that both in the control and GDM groups, the amount of any individual fatty acid was higher in maternal than in cord serum and no fatty acid was preferentially accumulated on the fetal side. For most fatty acids, the cord/maternal serum concentration ratio was similar in control and GDM groups except for AA and DHA (Fig. 2e, f), which were significantly lower in the GDM than the control group, despite the cord/maternal ratio of their respective precursors (GLA and ALA, respectively) being higher in the GDM than in the control group.
Cord/maternal serum fatty acid ratios of control (n=90) and GDM (n=84) samples. Data expressed as percentage (g/100 g fatty acids) of (a) SFA and MUFA, (b) n-6 and (c) n-3 fatty acids, and as concentration (mmol/l) of (d) SFA and MUFA, (e) n-6 and (f) n-3 fatty acids. Values were adjusted as described for Fig. 1. All fatty acids were log10-transformed for statistical comparisons. *p<0.05, **p<0.01, ***p<0.001 for cord vs maternal serum, as shown. All values are mean ± SEM; individual data point are shown
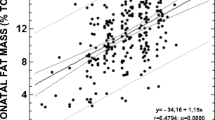
To test how differences in the placental transfer of AA and DHA could be implicated in the lower cord/maternal ratio observed for those two fatty acids in the GDM group, we analysed the correlation between their maternal and cord serum concentrations. Contrary to expectations, in the GDM group but not the control group, a significant and positive linear correlation between mother and fetus was found for both AA (Fig. 3a, b) and DHA (Fig. 3c, d). However, GDM decreased the ratio between those LCPUFA and their precursors in cord serum: AA/LA (1.22 ± 0.03 vs 1.13 ± 0.03, p < 0.05, control vs GDM, respectively) and DHA/ALA (50.1 ± 4.1 vs 25.9 ± 4.2, p < 0.0001, control vs GDM, respectively). That is, that the values were significantly lower in the GDM than the control group, which could be indicative of either a lower synthesis of these LCPUFA, a higher consumption of AA and DHA, or both, by fetuses affected by GDM compared with fetuses of control mothers. In this sense, we did not find any relationship between individual cord serum fatty acid concentration and the neonatal fat mass, probably owing to the fact that the offspring of pregnant women with GDM in this cohort were no larger or fatter than control participants.
Correlation between maternal and cord serum of AA and DHA concentrations in control (n=90) (a, c) and GDM (n=84) (b, d) samples. The regression line, 95% CI lines and prediction limit lines are shown, together with the Pearson correlation coefficient. All values were log10-transformed for statistical comparisons
Discussion
To the best of our knowledge, this is the first study to report a comparison of fatty acid profiles expressed as molar concentrations in maternal and cord serum of participants with and without GDM. Our results show that, in pregnancies affected by GDM, with satisfactory glycaemic control as a result of appropriate treatment with insulin or just with diet, the concentrations of saturated fatty acids (SFA), monounsaturated fatty acids (MUFA) and most PUFA in maternal serum at the end of pregnancy were lower than found in control pregnancies (Fig. 1), although the concentration of the two most important LCPUFA for fetal development, AA and DHA, showed no statistically significant differences between the groups. Moreover, in cord serum of women with GDM the concentrations of all fatty acids analysed were significantly lower than in control participants; in both groups, all fatty acids were present in lower concentrations in cord serum than in the respective maternal serum.
Disturbances of lipid metabolism under conditions of satisfactory glycaemic control have been associated with dysfunction in pregnancies affected by GDM [13, 31]. However, the impact of GDM on both maternal and fetal fatty acid metabolism is unclear. Our results show clear differences in fatty acid profiles between maternal serum (at term) and cord blood and between pregnancies affected by GDM and control pregnancies, when concentrations (mmol/l) rather than percentage distribution, is taken into consideration.
It is difficult to compare our findings with other studies because such studies do not appear to exist. In two previous studies, GDM was not associated with changes in the concentration of fatty acids evaluated in cord blood NEFA [19], phospholipids, cholesteryl esters or triacylglycerols [18]. However, the distribution of fetal fatty acids in the different lipid fractions is very dynamic as we found previously in rats [32], which can influence in the concentration of fatty acids present in a given lipid fraction at any given time, and therefore makes the correct interpretation of results more difficult. However, the fatty acid concentrations reported here represent the sum total of all fatty acids present in the different lipid fractions combined, therefore facilitating comparison between studies. To our knowledge, the study by Zhao et al [25] is the only one that analysed the effect of GDM on plasma concentrations of individual fatty acids, albeit only in maternal, not cord, samples; like us, they found a lower concentration of individual SFA, MUFA and PUFA circulating in plasma of GDM pregnant women with GDM compared with control participants, but differences did not reach statistical significance, probably owing to the differences in the sample size analysed (n = 24 in the GDM group vs n = 116 in the control group). Previously, Chen et al [20] reported that, in women with GDM, there is an increase in serum concentration of fatty acids compared with pregnant women without GDM, which contrasts with the results presented in our population. However, in that study the discrepancies may be attributed to the questionable method used to calculate the concentration of fatty acids, consisting of multiplying the percentage of each individual fatty acid by the total plasma NEFA concentration, as determined by an enzymatic method, so that the concentration of individual fatty acids was greater in the GDM group just because the NEFA concentration was higher than in the control group [20].
The underlying mechanism whereby, in pregnant women with optimal glycaemic control, GDM could reduce the fatty acid concentration in serum is unknown and, unfortunately, we can only speculate about it based on the concentration of fatty acids we found in maternal and cord serum. In a previous study designed to analyse the impact of GDM on fetal fatty acid metabolism [23], we observed that, in the umbilical venous plasma of newborns of mothers with GDM, the percentages of circulating fatty acids do not differ from those of controls, indicating that the placental transfer of fatty acids does not seem to be impaired under GDM conditions. In umbilical arterial plasma, however, a lower percentage of both AA and DHA and of total n-6 and total n-3 PUFA was apparent in GDM participants than in control participants, suggesting that the utilisation of those LCPUFA was increased by fetal tissues in GDM. In line with those results, the lower concentration of fatty acids found here in fetal serum of GDM compared with control participants could reflect an augmented utilisation of fatty acids by fetuses affected by GDM; moreover, this could generate a concentration gradient in favour of the fetus, which would explain the decrease in serum concentration of fatty acids observed in mothers with GDM. In similar circumstances, it has been reported that the placenta is able selectively to facilitate the transfer of those LCPUFA that are essential for nervous system development [33], in particular when fetal requirements are increased. This could cause the percentage of certain fatty acids, such as AA and DHA, to be higher in cord blood serum than in maternal serum, as found here and in agreement with other reports [24, 34, 35], and the term ‘biomagnification’ was coined for this proposed phenomenon [36]. However, based on our findings in fatty acids expressed as concentrations, the existence of such fetal biomagnification is not supported, because all fatty acids analysed are present in lower amounts in cord than in maternal serum, both in GDM and in the control condition, without GDM. It must be recognised that maternal GDM could exert a specific role on the availability of LCPUFA to the fetus as shown here by the significant positive correlation of the concentration of AA and DHA between maternal and cord serum in the GDM but not the control group, which would support a higher dependence on these fatty acids in fetuses of mothers with GDM. In the present study we did not observe any relationship between circulating insulin levels and individual fatty acid concentrations, neither in maternal nor in cord samples. However this finding is compatible with the possibility that higher concentrations of insulin in the cord serum of GDM neonates could stimulate the adipose tissue uptake of fetal fatty acids transported in TAG-VLDL, as we have previously described in fetuses affected by GDM and with high fat mass at delivery [15]. The lack of differences in neonatal fat mass in offspring of pregnant women with GDM vs control participants in this cohort could be masking such an effect.
It is not possible, however, to discard an alternative hypothesis to explain the effect of GDM on fatty acid concentration. It remains possible that it is a reduction in the ability to synthesise LCPUFA that explains the results. This was previously seen in pregnant women who developed gestational diabetes [37] and in experimental models of diabetes [38]. A further possibility is that GDM impairs placental transfer of LCPUFA, as observed in studies using stable isotopes [39, 40]. In this sense, the lower placental weight observed by us in the GDM group could be related to the reduced concentration of fatty acids in neonatal serum. However, we did not find any significant correlation between placental weight and any LCPUFA in the GDM cohort. Moreover, the birthweight/placental weight ratio used as an index to evaluate the placental efficiency [41] was similar in the control and GDM groups, indicating that the placental nutrient transfer capacity would not be affected in the GDM cohort. However, the possibility cannot be discarded that placental lipid metabolism, a factor that seems to be a determinant of placental transfer of fatty acids to the fetus [42, 43], could be influenced by GDM, affecting both placental fatty acid uptake by the placenta as well as the supply of fatty acids to the fetal circulation. Unfortunately, we can only explore those mechanisms based on the ratio between maternal/cord fatty acid concentrations in the first case, or product/precursor ratios in the latter. In fact, we found here that, in cord serum in the GDM group, the ratios AA/LA and DHA/ALA were lower than in the control group, indicating a lower ability for the synthesis of LCPUFA from their PUFA precursors in fetuses affected by GDM. Furthermore, we did not find differences in the cord/maternal serum concentration ratio for SFA and MUFA, or for LA, whereas the ratios for AA and DHA were statistically lower in the GDM than in the control group, supporting the possibility that placental LCPUFA transport is impaired in mothers with GDM, while the transport of the other fatty acids is not affected. It must be borne in mind, however, that in both cases those ratios could be influenced by the rate of disappearance of the product, which could lead to a misinterpretation of the results.
The strength of this study is the large sample size and the paired samples from maternal serum and umbilical cord arterial serum. However, weaknesses of the study include the fact that neither umbilical vein serum samples nor individual fatty acids in placental tissues were analysed, which impedes our understanding of the extent to which maternally derived fatty acids are taken up by the placenta and transferred to the fetus. Additionally, the GDM group included atypical characteristics, with no difference in neonatal fat mass compared with the control group, and with smaller placentas and a lower C-section rate than the control group. It is possible that the fact that all women in the GDM group had well controlled blood glucose, were normotensive, were not obese and had a BMI at delivery similar to that of the control group, have contributed to those outcomes. Moreover, samples from the GDM and control groups were recruited at different times, although all of them were preserved at −80°C until analysis, which was performed at the same time for all samples.
In conclusion, our data provide evidence that, contrary to the reported percentage values, serum concentrations of most fatty acids are lower in pregnancies affected by GDM than in control pregnancies, both in mothers and newborns, the only exceptions being maternal AA and DHA, which did not change between the two groups. Our findings of lower concentration values of both AA and DHA in fetal than in maternal serum do not support the use of the term ‘biomagnification’ for those two fatty acids in the fetal side. On the basis of the low cord/maternal fatty acid concentration ratios, which are even lower for both AA and DHA in the GDM than the control group, and because those two LCPUFA show a positive linear correlation between maternal and cord serum in the GDM but not the control group, it is proposed that there is a higher requirement for these maternal fatty acids in the fetuses of women with GDM than in those without GDM.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request. However, as this is a clinical dataset, the ability to share data is limited by patient confidentiality considerations.
Change history
01 April 2020
The authors regret that the incorrect figure was shown in Fig. 3d. The corrected figure is reproduced here.
Abbreviations
- AA:
-
Arachidonic acid (20:4 n-6)
- ALA:
-
α-Linolenic acid (18:3 n-3)
- C-section:
-
Caesarean section
- DGLA:
-
Dihomo-γ-linolenic acid (20:3 n-6)
- DHA:
-
Docosahexaenoic acid (22:6 n-3)
- EPA:
-
Eicosapentaenoic acid (20:5 n-3)
- GDM:
-
Gestational diabetes mellitus
- GLA:
-
γ-Linolenic acid (18:3 n-6)
- LA:
-
Linoleic acid (18:2 n-6)
- LCPUFA:
-
Long-chain polyunsaturated fatty acid(s)
- MUFA:
-
Monounsaturated fatty acid(s)
- OA:
-
Oleic acid (18:1 n-9)
- PA:
-
Palmitic acid (16:0)
- POA:
-
Palmitoleic acid (16:1)
- PUFA:
-
Polyunsaturated fatty acid(s)
- SA:
-
Stearic acid (18:0)
- SFA:
-
Saturated fatty acid(s)
- TAG:
-
Triacylglycerols
References
Herrera E, Ortega-Senovilla H (2016) Metabolism in normal pregnancy. In: Hod M, Jovanovic LG, Renzo GC, Leiva A, Langer O (eds) The textbook of pregnancy and diabetes. CRC, Florida, pp 17–27
Uauy R, Hoffman DR, Peirano P, Birch DG, Birch EE (2001) Essential fatty acids in visual and brain development. Lipids 36(9):885–895. https://doi.org/10.1007/s11745-001-0798-1
Innis SM (1991) Essential fatty acids in growth and development. Prog Lipid Res 30(1):39–103. https://doi.org/10.1016/j.placenta.2005.01.005
Herrera E, Ortega-Senovilla H (2015) The roles of fatty acids in fetal development. In: Duttaroy AK, Basak S (eds) Human placental trophoblasts Impact of maternal nutrition. CRC Press, Florida, pp 93–112
Alvino G, Cozzi V, Radaelli T, Ortega H, Herrera E, Cetin I (2008) Maternal and fetal fatty acid profile in normal and intrauterine growth restriction pregnancies with and without preeclampsia. Pediatr Res 64(6):615–620. https://doi.org/10.1203/PDR.0b013e31818702a2
Horvath A, Koletzko B, Szajewska H (2007) Effect of supplementation of women in high-risk pregnancies with long-chain polyunsaturated fatty acids on pregnancy outcomes and growth measures at birth: a meta-analysis of randomized controlled trials. Br J Nutr 98(2):253–259. https://doi.org/10.1017/S0007114507709078
Salem N Jr, Moriguchi T, Greiner RS et al (2001) Alterations in brain function after loss of docosahexaenoate due to dietary restriction of n-3 fatty acids. J Mol Neurosci 16(2–3):299–307. https://doi.org/10.1385/JMN:16:2-3:299
Mathiesen ER (2016) Pregnancy outcomes in women with diabetes-lessons learned from clinical research: The 2015 Norbert Freinkel Award Lecture. Diabetes Care 39(12):2111–2117. https://doi.org/10.2337/dc16-1647
Schaefer-Graf U, Napoli A, Nolan CJ, Diabetic Pregnancy Study Group (2018) Diabetes in pregnancy: a new decade of challenges ahead. Diabetologia 61(5):1012–1021. https://doi.org/10.1007/s00125-018-4545-y
Perna R, Loughan AR, Le J, Tyson K (2015) Gestational diabetes: long-term central nervous system developmental and cognitive sequelae. Appl Neuropsychol Child 4(3):217–220. https://doi.org/10.1080/21622965.2013.874951
Camprubi-Robles M, Campoy C, Garcia-Fernandez L, Lopez-Pedrosa JM, Rueda R, Martin MJ (2015) Maternal diabetes and cognitive performance in the offspring: a systematic review and meta-analysis. PLoS One 10(11):e0142583. https://doi.org/10.1371/journal.pone.0142583
Adane AA, Mishra GD, Tooth LR (2016) Diabetes in pregnancy and childhood cognitive development: a systematic review. Pediatrics 137(5):e20154234. https://doi.org/10.1542/peds.2015-4234
Herrera E, Ortega-Senovilla H (2010) Disturbances in lipid metabolism in diabetic pregnancy – Are these the cause of the problem? Best Pract Res Clin Endocrinol Metab 24(4):515–525. https://doi.org/10.1016/j.beem.2010.05.006
Schaefer-Graf UM, Graf K, Kulbacka I et al (2008) Maternal lipids as strong determinants of fetal environment and growth in pregnancies with gestational diabetes mellitus. Diabetes Care 31(9):1858–1863. https://doi.org/10.2337/dc08-0039
Ortega-Senovilla H, Schaefer-Graf U, Meitzner K, Abou-Dakn M, Herrera E (2013) Decreased concentrations of the lipoprotein lipase inhibitor angiopoietin-like protein 4 and increased serum triacylglycerol are associated with increased neonatal fat mass in pregnant women with gestational diabetes mellitus. J Clin Endocrinol Metab 98(8):3430–3437. https://doi.org/10.1210/jc.2013-1614
Thomas BA, Ghebremeskel K, Lowy C, Offley-Shore B, Crawford MA (2005) Plasma fatty acids of neonates born to mothers with and without gestational diabetes. Prostaglandins Leukot Essent Fatty Acids 72(5):335–341. https://doi.org/10.1016/j.plefa.2005.01.001
Zhao JP, Levy E, Fraser WD et al (2014) Circulating docosahexaenoic acid levels are associated with fetal insulin sensitivity. PLoS One 9(1):e85054. https://doi.org/10.1371/journal.pone.0085054
Leveille P, Ardilouze JL, Pasquier JC, Deacon C, Whittingstall K, Plourde M (2016) Fatty acid profile in cord blood of neonates born to optimally controlled gestational diabetes mellitus. Prostaglandins Leukot Essent Fatty Acids 115:48–52. https://doi.org/10.1016/j.plefa.2016.10.006
Stirm L, Kovarova M, Perschbacher S et al (2018) BMI-independent effects of gestational diabetes on human placenta. J Clin Endocrinol Metab 103(9):3299–3309. https://doi.org/10.1210/jc.2018-00397
Chen X, Scholl TO, Leskiw M, Savaille J, Stein TP (2010) Differences in maternal circulating fatty acid composition and dietary fat intake in women with gestational diabetes mellitus or mild gestational hyperglycemia. Diabetes Care 33(9):2049–2054. https://doi.org/10.2337/dc10-0693
Leveille P, Rouxel C, Plourde M (2018) Diabetic pregnancy, maternal and fetal docosahexaenoic acid: a review of existing evidence. J Matern Fetal Neonatal Med 31(10):1358–1363. https://doi.org/10.1080/14767058.2017.1314460
Min Y, Lowy C, Ghebremeskel K, Thomas B, Bitsanis D, Crawford MA (2005) Fetal erythrocyte membrane lipids modification: preliminary observation of an early sign of compromised insulin sensitivity in offspring of gestational diabetic women. Diabet Med 22(7):914–920. https://doi.org/10.1111/j.1464-5491.2005.01556.x
Ortega-Senovilla H, Alvino G, Taricco E, Cetin I, Herrera E (2009) Gestational diabetes mellitus upsets the proportion of fatty acids in umbilical arterial but not venous plasma. Diabetes Care 32(1):120–122. https://doi.org/10.2337/dc08-0679
Cinelli G, Fabrizi M, Rava L et al (2016) Influence of maternal obesity and gestational weight gain on maternal and foetal lipid profile. Nutrients 8(6):368–381. https://doi.org/10.3390/nu8060368
Zhao JP, Levy E, Shatenstein B et al (2016) Longitudinal circulating concentrations of long-chain polyunsaturated fatty acids in the third trimester of pregnancy in gestational diabetes. Diabet Med 33(7):939–946. https://doi.org/10.1111/dme.12978
Schaefer-Graf UM, Kjos SL, Fauzan OH et al (2004) A randomized trial evaluating a predominantly fetal growth-based strategy to guide management of gestational diabetes in Caucasian women. Diabetes Care 27(2):297–302. https://doi.org/10.2337/diacare.27.2.297
Schaefer-Graf UM, Meitzner K, Ortega-Senovilla H et al (2011) Differences in the implications of maternal lipids on fetal metabolism and growth between gestational diabetes mellitus and control pregnancies. Diabet Med 28(9):1053–1059. https://doi.org/10.1111/j.1464-5491.2011.03346.x
Catalano PM, Thomas AJ, Avallone DA, Amini SB (1995) Anthropometric estimation of neonatal body composition. Am J Obstet Gynecol 173(4):1176–1181. https://doi.org/10.1016/0002-9378(95)91348-3
Carpenter MW, Coustan DR (1982) Criteria for screening tests for gestational diabetes. Am J Obstet Gynecol 144(7):768–773. https://doi.org/10.1016/0002-9378(82)90349-0
Amusquivar E, Schiffner S, Herrera E (2011) Evaluation of two methods for plasma fatty acid analysis by GC. Eur J Lipid Sci Technol 113(6):711–716. https://doi.org/10.1002/ejlt.201000476
Barrett HL, Dekker Nitert M, McIntyre HD, Callaway LK (2014) Normalizing metabolism in diabetic pregnancy: is it time to target lipids? Diabetes Care 37(5):1484–1493. https://doi.org/10.2337/dc13-1934
Lopez-Luna P, Ortega-Senovilla H, Lopez-Soldado I, Herrera E (2016) Fate of orally administered radioactive fatty acids in the late-pregnant rat. Am J Physiol Endocrinol Metab 310(5):E367–E377. https://doi.org/10.1152/ajpendo.00449.2015
Crawford MA, Golfetto I, Ghebremeskel K et al (2003) The potential role for arachidonic and docosahexaenoic acids in protection against some central nervous system injuries in preterm infants. Lipids 38(4):303–315. https://doi.org/10.1007/s11745-003-1065-1
Schlormann W, Kramer R, Lochner A et al (2015) Foetal cord blood contains higher portions of n-3 and n-6 long-chain PUFA but lower portions of trans C18:1 isomers than maternal blood. Food Nutr Res 59:29348–29357. https://doi.org/10.3402/fnr.v59.29348
Luxwolda MF, Kuipers RS, Sango WS, Kwesigabo G, Dijck-Brouwer DA, Muskiet FA (2012) A maternal erythrocyte DHA content of approximately 6 g% is the DHA status at which intrauterine DHA biomagnifications turns into bioattenuation and postnatal infant DHA equilibrium is reached. Eur J Nutr 51(6):665–675. https://doi.org/10.1007/s00394-011-0245-9
Haggarty P (2002) Placental regulation of fatty acid delivery and its effect on fetal growth. A review. Placenta 23(Suppl A):S28–S38. https://doi.org/10.1053/plac.2002.0791
el Boustani S, Causse JE, Descomps B, Monnier L, Mendy F, Crastes de Paulet A (1989) Direct in vivo characterization of delta 5 desaturase activity in humans by deuterium labeling: effect of insulin. Metabolism 38(4):315–321. https://doi.org/10.1016/0026-0495(89)90117-0
Mimouni V, Poisson JP (1992) Altered desaturase activities and fatty acid composition in liver microsomes of spontaneously diabetic Wistar BB rat. Biochim Biophys Acta 1123(3):296–302. https://doi.org/10.1016/0005-2760(92)90010-s
Araujo JR, Correia-Branco A, Ramalho C, Keating E, Martel F (2013) Gestational diabetes mellitus decreases placental uptake of long-chain polyunsaturated fatty acids: involvement of long-chain acyl-CoA synthetase. J Nutr Biochem 24(10):1741–1750. https://doi.org/10.1016/j.jnutbio.2013.03.003
Larque E, Demmelmair H, Berger B, Hasbargen U, Koletzko B (2003) In vivo investigation of the placental transfer of (13)C-labeled fatty acids in humans. J Lipid Res 44(1):49–55. https://doi.org/10.1194/jlr.m200067-jlr200
Hayward CE, Lean S, Sibley CP et al (2016) Placental adaptation: what can we learn from birthweight:placental weight ratio? Front Physiol 7(28):1–13. https://doi.org/10.3389/fphys.2016.00028
Lewis RM, Wadsack C, Desoye G (2018) Placental fatty acid transfer. Curr Opin Clin Nutr Metab Care 21(2):78–82. https://doi.org/10.1097/MCO.0000000000000443
Perazzolo S, Hirschmugl B, Wadsack C, Desoye G, Lewis RM, Sengers BG (2017) The influence of placental metabolism on fatty acid transfer to the fetus. J Lipid Res 58(2):443–454. https://doi.org/10.1194/jlr.P072355
Acknowledgements
The authors wish to thank M. Morante of Universidad San Pablo-CEU for her excellent technical assistance, the midwives and obstetricians (especially M. Schmitter and A. Gonzales) of the Vivantes Medical Center for their support in recruiting participants and collecting the samples and P. F. Dodds (London, UK) for help in preparing the manuscript.
Funding
This study has been carried out with the financial support of the Spanish Ministry of Science and Innovation (SAF2012–39273) and Universidad San Pablo-CEU (MBS18C05).
Author information
Authors and Affiliations
Contributions
All authors were involved in the study design. HO-S performed the analysis and interpretation of data, statistical study and wrote the article; US-G collaborated in the acquisition of data, analysis of clinical histories and undertook review of the manuscript. EH contributed to manuscript preparation and reviewed the manuscript critically. All authors were involved in subsequent revisions and final approval. HO-S is the guarantor of this work.
Corresponding author
Ethics declarations
The authors declare that there is no duality of interest associated with this manuscript.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM
(PDF 453 kb)
Rights and permissions
About this article
Cite this article
Ortega-Senovilla, H., Schaefer-Graf, U. & Herrera, E. Pregnant women with gestational diabetes and with well controlled glucose levels have decreased concentrations of individual fatty acids in maternal and cord serum. Diabetologia 63, 864–874 (2020). https://doi.org/10.1007/s00125-019-05054-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-019-05054-x