Abstract
Study design
Retrospective cohort study.
Objectives
The goal of this study was to assess the impact of multidrug resistant gram-negative organisms (MDRGNOs) on outcomes in those with SCI/D.
Setting
VA SCI System of Care, Department of Veterans Affairs, United States.
Methods
Multidrug resistance (MDR) was defined as being non-susceptible to ≥1 antibiotic in ≥3 antibiotic classes. Multivariable cluster-adjusted regression models were fit to assess the association of MDRGNOs with 1-year mortality, 30-day readmission, and postculture length of stay (LOS) stratified by case setting patients. Only the first culture per patient during the study period was included.
Results
A total of 8,681 individuals with SCI/D had a culture with gram-negative bacteria during the study period, of which 33.0% had a MDRGNO. Overall, 954 (10.9%) died within 1 year of culture date. Poisson regression showed that MDR was associated with 1-year mortality among outpatients (IRR: 1.28, 95% CI, 1.06–1.54) and long-term care patients (OR: 2.06, 95% CI, 1.28–3.31). MDR significantly impacted postculture LOS in inpatients, as evidenced by a 10% longer LOS in MDR vs. non-MDR (IRR: 1.10, 95% CI, 1.02–1.19). MDR was not associated with increased 30-day readmission.
Conclusions
MDRGNOs are prevalent in SCI/D and MDR may result in poor outcomes. Further attention to prevention of infections, antibiotic stewardship, and management are warranted in this population.
Similar content being viewed by others
Introduction
The Centers of Disease Control and Prevention (CDC) estimate approximately two million illnesses and 23,000 deaths are caused by antibiotic resistant bacteria every year [1]. Specifically, multidrug resistant gram-negative organisms (MDRGNOs) have been a growing concern in healthcare and community settings [1]. These are particularly worrisome as MDRGNOs are often resistant to nearly all commonly used antibiotics. Gram-negative bacteria are frequently implicated in the most serious healthcare-associated infections (HAIs), with the common pathogens being Enterobacteriaceae, Pseudomonas aeruginosa, and Acinetobacter [1, 2].
Individuals with spinal cord injury or disorder (SCI/D) are at particularly high risk for infections due to frequent contact with the healthcare system, use of invasive medical devices, and antibiotic use [3,4,5]. Infections have been noted as a predictor of mortality and re-hospitalizations in individuals with SCI/D [6, 7]. In addition, previous research has found that individuals with SCI who have a hospital-acquired infection (HAI) have lowered survival [8].
Infections with MDRGNOs are associated with increased morbidity and mortality in general patient populations [9]. Gram-negative organisms are highly prevalent in individuals with SCI/D [10, 11], with increasing resistance over time [10]. Healthcare exposures, older age, exposure to antibiotics such as fluoroquinolones, carbapenems, cephalosporins, and sulfonamides, and comorbidities have been associated with MDRGNOs in those with SCI/D [10,11,12,13]. Furthermore, Fitzpatrick et al. found that although there were no differences in mortality rate, individuals with SCI/D colonized or infected with extended-spectrum β-lactamase (ESBL) bacteria had a longer postculture length of stay (LOS) than those with non-ESBL bacteria [5]. Additional research, focusing on MDR Acinetobacter in those with SCI/D did find increased risk of 30-day mortality in those with MDR cultures [13].
Therefore, the objective of this study was to assess the association between MDRGNOs and 1-year mortality, 30-day readmission, and postculture LOS in individuals with SCI/D stratified by case setting [inpatient, outpatient, long-term care (LTC)].
Methods
Study design, setting, and population
This was a retrospective cohort study of national Veterans Affairs (VA) medical encounter, microbiology, and pharmacy data from all 142 VA facilities. The first year of data (January 1, 2011 to December 31, 2011) was utilized to determine exposure for risk factors, and all microbiology culture data and analyses were based on the final 2 years of data collected January 1, 2012 to December 31, 2013. Veterans with SCI/D seen at a VA medical center during the study period were included. Veterans with multiple sclerosis and amyotrophic lateral sclerosis were excluded as these conditions are not associated with stable nonprogressive spinal cord neurological deficits and therefore not the focus of the VA SCI/D system of care.
Data sources and definitions
Several national VA datasets were utilized for this study. Patient demographics, healthcare utilization, facility information, microbiology, and pharmacy data were obtained from the VA Corporate Data Warehouse (CDW). The CDW is a national repository that is updated daily and includes clinical and administrative data from the Veterans Health Administration (VHA). The VA Vital Status File contains dates of death combined from the Veterans Benefits Administration Beneficiary Identification and Records Location System death file, the VA Medicare Vital Status File, and the Social Security Administration Death Master File, and was used to obtain mortality data. SCI/D characteristics were collected from the VA SCD registry which is a national database that contains spinal cord injury information derived from patient registries.
Demographic data (age, sex, race/ethnicity, and comorbidities) were identified 1 year prior to and during the visit or admission the culture was identified. Specimen type was categorized into blood, urine, sputum, and other (wound, tissue, body fluid, and bone cultures). ICD-9 codes from the Deyo-Charlson co-morbidity index [14], including pressure ulcer which is a common condition in SCI/D individuals, were used to identify comorbidities. Duration of SCI was categorized between 0–10 years, 11–20 years, and 21+ years. Healthcare exposure was defined as exposures in the previous 90 days prior to culture date (long-term care, intensive care unit stay, surgery, mechanical ventilation, and previous healthcare admission). Antibiotic and steroid exposures were defined as receipt of the medication in the 90 days before culture date. The VA utilizes a hub and spoke system where 24 VA facilities are designated ‘hubs’ or SCI centers delivering specialized SCI care. ‘Hub’ centers are connected to ‘spoke’ facilities; spoke facilities provide community-based care for SCI/D individuals. Region was defined using the US Census Bureau regions; San Juan, Puerto Rico, and Manila, Philippines were grouped into the South region.
Multiple cultures from the same patient within 30 days were excluded as well as cultures that did not grow at least one gram-negative bacteria. Only the first culture per patient that grew a gram-negative bacteria during the study period was included. All gram-negative isolates were identified as MDR or non-MDR based on definitions from Magiorakos et al. which accounted for intrinsic resistance [15]. The sample was limited to cultures with antibiotic sensitivities and any culture with at least one MDR isolate was considered a MDRGNO culture.
1-year mortality was defined as death within 1 year from the date of culture. 30-day hospital readmission was defined as readmission of the patient with 30 days of the culture date and only applicable among those with an initial culture collected in the inpatient setting. Post-culture LOS was defined as time from date of culture to date of discharge and was also only applicable among those in the inpatient setting.
Statistical analysis
All analyses were stratified by care setting (inpatient, outpatient, and LTC). LTC included individuals in skilled nursing facilities and rehabilitation centers. Bivariate analysis was conducted and unadjusted odds ratios (ORs) and incidence rate ratios (IRRs) and 95% confidence intervals (CIs) were estimated to identify risk factors for outcomes related to MDRGNOs. Cluster-adjusted logistic regression models were used for outcomes related to 1-year mortality in inpatients and LTC patients and 30-day readmission in inpatients. Multivariable cluster-adjusted multilevel mixed effects Poisson regression models were fitted to assess the association of MDRGNO with 1-year mortality among outpatients. A fixed-effects, cluster-adjusted, negative-binomial model was used to assess post-admission LOS among inpatients. Logistic models were used because the outcome (mortality and 30-day readmission) occurred in >10% of the cohort among inpatients and LTC patients. Poisson regression was selected for the outpatient 1-year mortality analysis as the event was rare [16]. Finally, LOS was modeled as a count variable using a negative-binomial model instead of a Poisson model because the outcome was overdispersed. All models were population-averaged cluster adjusted by individual facility to account for facility variations. Variables were included in multivariable regression if they were significant in the unadjusted analyses, and the most parsimonious or simplest model was selected. Adjusted ORs and 95.0% CIs and IRRs and 95.0% CIs were reported for all regression models. A p value of ≤0.05 was considered statistically significant. All analyses were performed using STATA software version 14.2 (StataCorp, College Station, TX).
Results
Overview
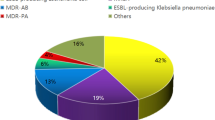
There was a total of 8,681 individuals with SCI/D who had a gram-negative bacteria grow in culture during the study period. The sample had a mean age of 62.1 (sd = 13.2) years, SCI duration of 19.4 (sd = 15.5) years, and Charlson co-morbidity score of 2.3 (sd = 2.0). Most of the cohort was White (63.2%), male (96.7%), from the South (51.1%), and about half the cohort had tetraplegia (55.2%). Most specimens were urine cultures (88.7%) and 20% of patients had a polymicrobial culture. The majority of patients were outpatients (n = 6111, 70.4%). Inpatients comprised 25.1% of the total cohort (n = 2180) and the remaining were LTC patients (n = 390, 4.5%). From the overall sample, 2866 of gram-negative cultures (33.0%) were MDR. Overall, 40.0% (n = 875) of inpatient cultures, 30.1% (n = 1,841) of outpatient cultures, and 38.4% (n = 150) of LTC patient gram-negative cultures were MDR.
Overall, 11.0% of the cohort (n = 954) died within 1 year of culture date (13.4% of the MDRGNOs versus 9.8% of non-MDRGNOs). Of those who died in 1 year, 456 (47.8%) were outpatients, 391 (41.0%) were inpatients, and 107 (11.2%) were LTC patients. Overall, median postculture LOS in inpatients was 25 (IQR = 82) days (MDRGNO [37; IQR: 111] vs non-MDRGNO [21; IQR: 71]). A total of 297 inpatients (13.6%) experienced a readmission within 30 days of culture date (14.9% of the MDRGNOs vs 12.9% of the non-MDRGNOs).
Bivariate unadjusted results
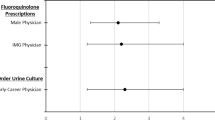
Bivariate unadjusted results for showed that having an MDRGNO was not associated with 1-year mortality in inpatients (OR: 1.15, 95% CI, 0.87-1.52) (Table 1). However, having an MDRGNO was associated with increased 1-year mortality in outpatients (IRR: 1.28, 95% CI, 1.06–1.54) and among LTC patients (OR = 2.06, 95% CI, 1.28–3.31). Among inpatients, risk factors for 1-year mortality included increased age, White race (vs. Black race), SCI characteristics, not being at an SCI center, blood or other specimen types (vs. urine), comorbidities, healthcare stay, and antibiotics or steroids in the previous 90 days before culture. For outpatients, similar risk factors were found for mortality; in addition male sex, north region (vs. south), increased duration of injury, having a polymicrobial culture, surgery, and mechanical ventilation were found to be risk factors for outpatients. Risk factors for LTC patients included increased age, having COPD or tumor or cancer, and use of chronic steroids. Individual exposure data in the previous 90 days is described in Fig. 1. The most frequent antibiotics prescribed were quinolones, extended-spectrum penicillins, and sulfonamides for inpatient and LTC groups. Among outpatients, the most frequently prescribed antibiotics were nitrofurantoins, quinolones, and sulfonamides. Carbapenems, third/fourth generation cephalosporins, and extended-spectrum penicillins increased the odds of inpatient mortality while quinolones, third/fourth generation cephalosporins, and extended-spectrum penicillins increased the risk of mortality in outpatients. No antibiotics were associated with increased risk of mortality in LTC individuals.
Proportion of inpatients, outpatients, and LTC individuals that received the type of antibiotic 90 days before and association with outcomes of mortality, readmission, and length of stay. Asterisk denotes unadjusted OR or unadjusted IRR were significant and the proportion of those that received the specific antibiotic were different among those that died and did not die. White circle denotes unadjusted OR was significant and the proportion of those that received the specific antibiotic were different among those that were readmitted and were not readmitted. Plus symbol denotes unadjusted IRR was significant and the length of stay differed for those that received the specific antibiotic compared with those that did not.
Having an MDRGNO was not associated with increased 30-day readmission in inpatients (OR: 1.23, 95% CI, 0.96–1.57) (Table 2). However, other factors associated with 30-day readmission in inpatients included not being at an SCI center, having a blood or other specimen (vs urine), having liver disease, and having a healthcare stay or antibiotic exposure in the previous 90 days before culture. Quinolones, third/fourth generation cephalosporins, carbapenems, and extended-spectrum penicillins exposure all increased risk of 30-day readmission (Fig. 1, Table 2).
In the bivariate analysis, MDR was associated with increased postculture LOS in inpatients (IRR: 1.38, 95% CI, 1.37–1.39). Other risk factors for inpatients include male sex, Black race, northeast region, having tetraplegia or traumatic injury, increased duration of injury, other specimen types, having a pressure ulcer, COPD, cerebrovascular disease, diabetes, liver disease, tumor or cancer, not using chronic steroids, and having an LTC stay, not having surgery, not having mechanical ventilation, not having a genitourinary procedure, not having healthcare admission, or antibiotics in the previous 90 days before culture. Specific antibiotics associated with increased LOS were sulfonamides, carbapenems, and tetracyclines while nitrofurantoins, quinolones, and extended-spectrum penicillins were associated with a decreased LOS.
Multivariable regression results
In multivariable regression, after adjustment with other covariates, results showed that having an MDRGNO culture was not associated with 1-year mortality in inpatients (OR:1.15, 95% CI, 0.87–1.52) but was associated with increased risk of 1-year mortality among outpatients (IRR: 1.28, 95% CI, 1.06–1.54) and increased odds of 1-year mortality in LTC patients (OR: 2.06, 95% CI, 1.28–3.31) (Table 3). An MDRGNO culture was not associated with increased 30-day readmission in inpatients (OR: 1.25, 95% CI, 0.97–1.61) but was associated with increased LOS among inpatients (IRR: 1.10, 95% CI, 1.02–1.19) (Table 4).
Discussion
The purpose of this study was to understand how MDRGNOs impact outcomes in Veterans with SCI/D. Our results showed that having an MDRGNO culture was associated with 1-year mortality in outpatients and LTC patients. Furthermore, although MDR did not affect 30-day readmission among inpatients, it was significantly associated with increased LOS.
Overall, 1 in 10 Veterans with SCI/D with a gram-negative culture died within 1-year of the culture date. We found that outpatients with MDRGNOs had 27% increased risk for 1-year mortality. Outpatients and LTC patients who died within 1-year of MDRGNO culture were older and sicker, with outpatients having had prior healthcare exposures. It is possible that outpatients with MDR may have substantial healthcare exposures, possibly for a chronic condition that may shorten life expectancy. However, our data showed that outpatients had a lower mean charlson score (1.2 (sd = 1.5)) compared with inpatients (3.0 (sd = 1.9)). It is unclear what is causing the increased risk for mortality in outpatients. It is also important to consider that MDRDNOs may be markers for increased mortality but may not be the cause of death. This is consistent with findings that those with increased risk for MDRGNOs were sicker and had prior healthcare exposures.
In addition, though we found no effect of 30-day readmission in inpatients, LOS for inpatients with MDRGNOs was significantly greater compared with those without MDRGNOs. Having an MDRGNO was associated with a 10.0% longer postculture LOS among inpatients in this study. Previously, having SCI has been found to be a unique predictor for increased LOS after HAI infection among those in a rehabilitation unit [17]. Furthermore, our results are also consistent with other published findings that nosocomial infections and MDR infection in those with SCI/D result in increased LOS [8, 18, 19]. However, as noted in the case of mortality, MDR may be a marker for increased LOS and may be a part of a combination of factors that increase postculture LOS.
There were several limitations within this study. First, our study did not distinguish between infection and colonization, specifically with urinary colonization. This is an important limitation, as including patients who were only colonized rather than actively infected may have masked or blunted the actual association reported in this study. Second, we did not capture data from healthcare external to the VA which may bias results if there are unique characteristics associated with those that receive care outside the VA. Finally, because our study focused on Veterans with SCI/D who receive care at the VA and are mostly male, the results of this study may not be generalizable to other populations with SCI/D. This study however, has the advantage that it reports on data from the VHA, the single largest integrated healthcare system in the United States that is also the largest provider of care for patients with SCI/D.
In summary, among a large national cohort of individuals with SCI/D and cultures with gram-negative organisms, having an MDRGNO was associated with increased risk and increased odds for 1-year mortality among outpatients and LTC patients, respectively. Furthermore, among inpatients, having an MDRGNO was not associated increased postculture LOS but not 30-day hospital readmission. It is important for clinicians to be aware of the increased risk for poor outcomes in patients with SCI/D and MDRGNOs. Results from this study support increased attention to antibiotic stewardship in SCI/D populations to prevent the development of antibiotic resistance in gram-negative bacteria and effective infection prevention strategies to prevent transmission of MDRGNOs.
Data archiving
The datasets generated during and/or analyzed during the current study are not publicly available as the sample size is too large to obtain informed consents and HIPAA authorizations for public discloser of the final study data containing PII and/or PHI and would be inconsistent with the IRB approved waiver of informed consent and waiver of HIPPA authorization that was approved.
References
Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States. Atlanta, Georgia: Centers for Disease Control and Prevention; 2013. https://www.cdc.gov/drugresistance/threat-report-2013/pdf/ar-threats-2013-508.pdf. Accessed 7 Mar 2019.
Weiner LM, Webb AK, Limbago B, Dudeck MA, Patel J, Kallen AJ, et al. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2011–2014. Infect Control Hosp Epidemiol. 2016;37:1288–301.
Montgomerie JZ. Infections in patients with spinal cord injuries. Clin Infect Dis. 1997;25:1285–90.
Evans CT, LaVela SL, Weaver FM, Priebe M, Sandford P, Niemiec S, et al. Epidemiology of hospital-acquired infections in veterans with spinal cord injury and disorder. Infect Control Hosp Epidemiol. 2008;29:234–42.
Fitzpatrick MA, Suda KJ, Safdar N, Goldstein B, Jones MM, Poggensee L, et al. Unique risks and clinical outcomes associated with extended-spectrum betalactamase Enterobacteriaceae in veterans with spinal cord injury/disorder: a case-case-control study. Infect Control Hosp Epidemiol. 2016;37:768–76.
Rabadi MH, Mayanna SK, Vincent AS. Predictors of mortality in veterans with traumatic spinal cord injury. Spinal Cord. 2013;51:784–8.
DeJong G, Tian W, Hsieh CH, Junn C, Karam C, Ballard PH, et al. Rehospitalization in the first year of traumatic spinal cord injury after discharge from medical rehabilitation. Arch Phys Med Rehabil. 2013;94:S87–97.
LaVela SL, Evans CT, Miskevics S, Parada JP, Priebe M, Weaver FM. Long-term outcomes from nosocomial infections in persons with spinal cord injuries and disorders. Am J Infect Control. 2007;35:393–400.
Nelson RE, Slayton RB, Stevens VW, Jones MM, Khader K, Rubin MA, et al. Attributable mortality of healthcare-associated infections due to multidrug-resistant gram-negative bacteria and methicillin-resistant staphylococcus aureus. Infect Control Hosp Epidemiol. 2017;38:848–56.
Fitzpatrick MA, Suda KJ, Safdar N, Burns SP, Jones MM, Poggensee L, et al. Changes in bacterial epidemiology and antibiotic resistance among Veterans with spinal cord injury/disorder over the past 9 years. J Spinal Cord Med. 2018;41:199–207.
Evans CT, Fitzpatrick MA, Jones MM, Burns SP, Poggensee L, Ramanathan S, et al. Prevalence and factors associated with multidrug-resistant gram-negative organisms in patients with spinal cord injury. Infect control Hosp Epidemiol. 2017;38:1464–71.
Kale IO, Fitzpatrick MA, Suda KJ, Burns SP, Poggensee L, Ramanathan S, et al. Risk factors for community-associated multidrug-resistant Pseudomonas aeruginosa in veterans with spinal cord injury and disorder: a retrospective cohort study. Spinal Cord. 2017;55:687–91.
Ramanathan S, Suda KJ, Fitzpatrick MA, Poggensee L, LaVela SL, Burns SP, et al. Multidrug-resistant Acinetobacter: Risk factors and outcomes in veterans with spinal cord injuries and disorders. Am J Infect Control. 2017;45:1183–9.
Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83.
Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18:268–81.
Agresti A. Categorical data analysis. 3rd ed. Hoboken, New Jersey: John Wiley & Sons; 2013.
Mylotte JM, Graham R, Kahler L, Young BL, Goodnough S. Impact of nosocomial infection on length of stay and functional improvement among patients admitted to an acute rehabilitation unit. Infect Control Hosp Epidemiol. 2001;22:83–7.
Giske CG, Monnet DL, Cars O, Carmeli Y. ReAct-action on antibiotic resistance. clinical and economic impact of common multidrug-resistant gram-negative bacilli. Antimicrob Agents Chemo. 2008;52:813–21.
Patel G, Huprikar S, Factor SH, Jenkins SG, Calfee DP. Outcomes of carbapenem-resistant Klebsiella pneumoniae infection and the impact of antimicrobial and adjunctive therapies. Infect Control Hosp Epidemiol. 2008;29:1099–106.
Funding
This work was supported by funding from the Veterans Health Administration, Office of Research and Development, Rehabilitation Research and Development Service SPIRE Award (grant no. B-1583-P). The views expressed in this article are those of the authors and do not necessarily reflect the policy of the Department of Veterans Affairs or the US government.
Author information
Authors and Affiliations
Contributions
CTE was the principal investigator and responsible for overseeing the project that the data were obtained from. CT also provided guidance and expertize in epidemiological methods and reviewing the manuscript. SR was responsible for data analysis and writing the manuscript. MAF and KJS provided helped with writing the discussion and providing infectious disease expertize. SPB and SLV provided spinal cord injury expertise related to the paper and findings. MMJ provided clinical experience with VA microbiology data and infectious disease expertize. All authors provided edits and revisions of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical statement
This study was approved by the Edward Hines Jr. VA Hospital IRB. We certify that all applicable institutional and governmental regulations concerning the ethical use of human subjects data were followed during the course of this research.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ramanathan, S., Fitzpatrick, M.A., Suda, K.J. et al. Multidrug-resistant gram-negative organisms and association with 1-year mortality, readmission, and length of stay in Veterans with spinal cord injuries and disorders. Spinal Cord 58, 596–608 (2020). https://doi.org/10.1038/s41393-019-0393-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41393-019-0393-y
This article is cited by
-
The prevalence of antibiotic-resistant and multidrug-resistant bacteria in urine cultures from inpatients with spinal cord injuries and disorders: an 8-year, single-center study
BMC Infectious Diseases (2022)
-
Epidemiology and outcomes associated with carbapenem-resistant Acinetobacter baumannii and carbapenem-resistant Pseudomonas aeruginosa: a retrospective cohort study
BMC Infectious Diseases (2022)
-
Multidrug-resistant bacteria in urine culture among patients with spinal cord injury and disorder: Time to first detection and analysis of risk factors
Spinal Cord (2022)