Abstract
The presence of mixed 6-thioguanine-nonionic surfactant adsorption layers affects the mechanism and kinetics of the irreversible Bi(III) ion electroreduction process in chlorates(VII). Tween 80 and Triton X-100 change the dynamics of the catalytic effects of 6-thioguanine on Bi(III) ion electroreduction with the tendency of inhibition. The mechanism of catalytic activity of 6-thioguanine is associated with formation of complexes under specific conditions which exist on the electrode surface. The effects of both 6TG and suitable surfactant mixture on the first electron transfer are much greater than those on the transition of other electrons. The Bi-6TG type complex plays the main role in the Bi-6-thioguanine-Tween 80 or Bi-6-thioguanine-Triton X-100 systems, as 6-thioguanine dominates in the formation of adsorption equilibria of the studied mixtures.

The reaction path obtained for Bi(III) ion electroreduction showing active complexes Bi-6TG acting as intermediates in electron transfer and in the presence of surfactants.
Similar content being viewed by others
Introduction
Knowledge of organic substance effects on the electrode reaction rate is of significant importance in the elaboration of technological or pharmacological characteristics. It was found that the ability of the organic substance, undergoing adsorption on the electrode, to form complexes with the depolarizer in the near-electrode layer and location of the reduction potential of the depolarizer in the area of labile adsorption equilibrium of the organic substance [1,2,3]. A significant effect of activity of water and some polarographically active amino acids on the mechanism and kinetics of Bi(III) ion electroreduction [4, 5] was proven which developed the interpretation of the “cap-pair” effect mechanism. The results of previous studies pointed out explicitly to the key role of active complexes taking part in electron transition in the electrode process. Further studies of the kinetics and mechanism of Bi(III) ion electroreduction oriented towards the changes of process dynamics affected by formation of the mixed adsorption 6-mercaptopurine-Tween 80 and 6-mercaptopurine-Triton X-100 layers seemed to be extremely interesting taking into account a practical aspect of an investigating mechanism, e.g., action of drugs [6]. Thus, expanding the subject including other drugs applied in antitumorous chemotherapy is justified.
6-thioguaine (6TG) is an example of natural antimetabolites of purine bases and is used in antitumorous chemotherapy mainly for treatment of acute leukemias as well as in consolidation and maintenance of remission of acute and chronic marrow leukemia and acute lymphoblastic leukemia. 6TG inhibits various stages of angiogenesis and also tumor growth [7, 8]
The presented paper is part of a broader project concerning the influence of mixed adsorption layers of 6-thioguanine-Tween 80 as well as 6-thioguanine-Triton X-100 on the kinetics and mechanism of the multi-step process of Bi(III) electroreduction in chlorates(VII).
The studies carried out using the electrochemical method measurements such as DC polarography, square wave voltammetry (SWV), and cyclic voltammetry (CV), as well as electrochemical impedance spectroscopy, will allow attempting to examine and understand the interactions between a chosen drug, depolarizer, and surfactant (which can be finally used in a potential targeted therapy).
Experiment
All electrochemical experiments were carried out with a μAutolab FRA2/GPES (version 4.9) analyzer (Eco Chemie, Utrecht, Netherlands). The working electrode was a dropping or hanging mercury electrode with a controlled increase rate and a constant drop surface (0.0116241 cm2) made by MTM, Poland. The reference was a silver chloride electrode and the auxiliary electrode was platinum.
To prepare solutions, the following analytically pure reagents were applied: Bi(NO3)3·5H2O (Fluka), NaClO4 (Fluka), HClO4 (Fluka), 6-thioguanine (Fluka), Triton X-100 (Sigma-Aldrich), and Tween 80 (Sigma-Aldrich). The depolarizer role was played by Bi(III) (1 × 10−3 mol ∙ dm−3 Bi(III)) ions, and 2 mol ∙ dm−3 chlorate(VII) solution was the basic electrolyte. Preliminary studies have shown the greatest catalytic effect of 6-thioguanine was observed in 2 mol ∙ dm−3 chlorate(VII) solution.
6-Thioguanine in the concentration range from 0.1 to 10 × 10−3 mol ∙ dm−3 was used as a catalyst of the Bi(III) ion electroreduction process. However, the change of dynamics of electrode process kinetics was obtained using the nonionic surfactants: Triton X-100 and Tween 80 for 1 × 10−6 to 1 × 10−3 mol ∙ dm−3 concentrations.
The solutions were deoxygenated with high-purity nitrogen prior to each experiment and kept in the nitrogen atmosphere during the measurements at 298 K. Water applied to prepare all solutions was purified in the Millipore system.
The details of the kinetic parameter determination are described elsewhere [9].
Results and Discussion
Introduction of 6-thioguanine to the solutions of Bi(III) ions in 2 mol ∙ dm−3 chlorates(VII) causes the increase in SWV peak current of Bi(III) ion electroreduction (Figs. 1 and 2 6-thioguanine (○)) and a simultaneous decrease in the peak width in the middle of its height. The magnitude of these effects depends on the 6TG concentration. However, the addition to the basic electrolyte containing already 6TG, Tween 80 or Triton X-100 results in the decrease in currents of SWV peaks (Figs. 1 and 2 and Tables 1 and 2). With the increasing concentration of surfactants, currents of peaks decrease which suggests their inhibiting effect on the electrode process rate.
The SWV peaks of 1 × 10−3 mol ∙ dm−3 Bi(III) electroreduction in 2 mol ∙ dm−3 chlorates(VII) (♦) in the presence of 1 × 10−3 mol ∙ dm−3 6-thioguanine ( ) and 1 × 10−3 mol ∙ dm−3 6-thioguanine with Tween 80. The concentration of Tween 80 in 10−5 mol ∙ dm−3: (
) and 1 × 10−3 mol ∙ dm−3 6-thioguanine with Tween 80. The concentration of Tween 80 in 10−5 mol ∙ dm−3: ( ) 5, (
) 5, ( ) 10, (
) 10, ( ) 20, (
) 20, ( ) 30. Operational parameters: pulse amplitude 20 mV, frequency 120 Hz and step potential 2 mV.
) 30. Operational parameters: pulse amplitude 20 mV, frequency 120 Hz and step potential 2 mV.
The SWV peaks of 1 × 10−3 mol ∙ dm−3 Bi(III) electroreduction in 2 mol ∙ dm−3 chlorates(VII) (♦) in the presence of 1 × 10−3 mol ∙ dm−3 6-thioguanine ( ) and 1 × 10−3 mol m−3 6-thioguanine with Triton X-100. The concentration of Triton X-100 in 10−5 mol ∙ dm−3: (
) and 1 × 10−3 mol m−3 6-thioguanine with Triton X-100. The concentration of Triton X-100 in 10−5 mol ∙ dm−3: ( ) 5, (
) 5, ( ) 10, (
) 10, ( ) 15, (
) 15, ( ) 20, and (
) 20, and ( ) 25. Operational parameters: pulse amplitude 20 mV, frequency 120 Hz, and step potential 2 mV
) 25. Operational parameters: pulse amplitude 20 mV, frequency 120 Hz, and step potential 2 mV
Such qualitative estimation of the effect of the studied substances on the electroreduction process of Bi(III) ions in 2 mol ∙ dm−3 chlorates(VII) is confirmed by the results obtained from the CV curves (Fig. 3a, b). The presence of 1 × 10−3 mol ∙ dm−3 6-thioguanine in a solution of basic electrolyte results in a decrease in the distance between the anodic and cathodic peaks (∆Eac) in comparison with the distance registered for the solution of Bi(III) ions in all chlorates(VII) which confirms the increase in reversibility of the Bi(III) ion electroreduction process.
The cyclic voltammograms of 1 × 10−3 mol ∙ dm−3 Bi(III) in 2 mol ∙ dm−3 chlorates(VII) ( ) in the presence of 1 × 10−3 mol ∙ dm−3 6-thioguanine (
) in the presence of 1 × 10−3 mol ∙ dm−3 6-thioguanine ( ) (
) ( ) and 1 × 10−3 mol ∙ dm−3 6-thioguanine with 4 × 10−5 mol ∙ dm−3 Tween 80 (
) and 1 × 10−3 mol ∙ dm−3 6-thioguanine with 4 × 10−5 mol ∙ dm−3 Tween 80 ( ) (a) and 5 × 10−5 mol ∙ dm−3 Triton X-100 (
) (a) and 5 × 10−5 mol ∙ dm−3 Triton X-100 ( ) (b). Operational parameters: scan rate 50 mV s−1 and step potential 5 mV
) (b). Operational parameters: scan rate 50 mV s−1 and step potential 5 mV
With an increase in the concentration of both Tween 80 and Triton X-100, the ∆Eac increase which indicates slowing down of the electrode process is observed [10]. Similar dependences were observed for the Bi-6-mercaptopurine-Tween 80 and Bi-6-mercaptopurine-Triton X-100 [6]. This was explained by the formed mixed adsorption layers whose presence changed evidently the dynamics of the Bi(III) ion electroreduction process rate [11, 12].
Slight changes with a change in the rate of electrode polarization (particularly at low polarization rates (Fig. 4a, b)) indicate that the stage controlling the rate of Bi(III) ion electroreduction in the presence of 6TG is a chemical reaction. This reaction seems to be formation of active complexes of Bi-6TG which act as agents in electron transfer on the electrode surface [4]. It should be emphasized that independent of complex-forming properties of the catalyzing substance, its adsorption on the electrode [4] shifts the Bi(III) ion complexation equilibrium favorably. Moreover, Bi(III) ion reduction proceeds in the area of specifically adsorbed 6-thioguanine [6].
The influence of polarization rate on the difference between the potentials of the anodic and cathodic peaks ∆Eac for the Bi(III)/Bi(Hg) couple in 2 mol ∙ dm−3 chlorates(VII) ( ) in the presence of 1 × 10−3 mol ∙ dm−3 6-thioguanine (
) in the presence of 1 × 10−3 mol ∙ dm−3 6-thioguanine ( ) (
) ( ) and 1 × 10−3 mol ∙ dm−3 6-thioguanine with 10−5 mol ∙ dm−3 Tween 80: (
) and 1 × 10−3 mol ∙ dm−3 6-thioguanine with 10−5 mol ∙ dm−3 Tween 80: ( ) 5, (
) 5, ( ) 10, (
) 10, ( ) 20, (
) 20, ( ) 30, and (■) 80 (a), and with 10−5 mol ∙ dm−3 Triton X-100: (
) 30, and (■) 80 (a), and with 10−5 mol ∙ dm−3 Triton X-100: ( ) 5, (
) 5, ( ) 10, (
) 10, ( ) 15, (
) 15, ( ) 20, and (
) 20, and ( ) 25 (b). Operational parameters: scan rate 5–1000 mV s−1 and step potential 5 mV
) 25 (b). Operational parameters: scan rate 5–1000 mV s−1 and step potential 5 mV
The active Bi-6-thioguanine complexes are therefore located within the adsorption layer [13].
The previous research on the kinetics of Bi(III) ion electroreduction in chlorates(VII) in the presence of amino acids [4] indicates the existence of such active complexes at the electrode. The presence of these complexes was not observed by the use of a spectrophotometry method.
The effect of accelerating Bi(III) electroreduction by 6-thioguanine is a result of very effective blending of orbitals of the Bi-6TG complexes in comparison with Bi(III) ions. Therefore, formation of the complex changes the orbital structure of the acceptor facilitating transition of electrons in the electroreduction process [14, 15].
In turn, the effect of mixed adsorption layers of 6-thioguanine-Tween 80 and 6-thioguanine-Triton X-100 on the electrode process is proven by the evident changes of ∆Eac values with the polarization rate change (Fig. 4a and b), particularly above the concentrations 5 × 10−4 mol ∙ dm−3 for Tween 80 and 1 × 10−4 mol ∙ dm−3 for Triton X-100 (CMC). This suggests also changes in the mechanism of the Bi(III) ion electroreduction process in the presence of 6TG and surfactant mixture in the basic electrolyte solution.
Figure 5a and b present the DC polarograms of electroreduction of 1 × 10−3 mol ∙ dm−3 Bi(III) in 2 mol ∙ dm−3 chlorates(VII) and in the presence of 1 × 10−3 mol ∙ dm−3 6TG as well as the increasing surfactant concentration. The addition of 6TG to the solution changes the DC wave height of Bi(III) ion electroreduction which changes its inclination. The increase in DC wave inclination confirms increasing reversibility of the electrode process in the presence of 6-thioguanine. However, the addition of surfactants to the basic electrolyte solution changes the DC wave inclination in the opposite indicating the change in the dynamics of 6TG catalytic activity in the Bi(III) ion electroreduction process towards inhibition. Moreover, with the increasing concentration of surface active substances, the height of DC waves decreases resulting in the changes of values of diffusion coefficients Dox of the depolarizer ions in the solution (e.g., DoxBi = 5.0 × 10−6 cm2 s−1, \( {D}_{\mathrm{oxBi}+1\times {10}^{-3}\ 6\mathrm{TG}}=1.88\times {10}^{-6}\ {\mathrm{cm}}^2\ {\mathrm{s}}^{-1} \), \( {D}_{\mathrm{oxBi}+1\times {10}^{-3}\ 6\mathrm{TG}+5\times {10}^{-5}\ \mathrm{Tween}\ 80}=4.36\times {10}^{-7}\ {\mathrm{cm}}^2\ {\mathrm{s}}^{-1} \), \( {D}_{\mathrm{oxBi}+1\times {10}^{-3}\ 6\mathrm{TG}+1\times {10}^{-4}\ \mathrm{Tween}\ 80}=3.16\times {10}^{-7}\ {\mathrm{cm}}^2\ {\mathrm{s}}^{-1} \), and \( {D}_{\mathrm{oxBi}+1\times {10}^{-3}\ 6\mathrm{TG}+2\times {10}^{-4}\ \mathrm{Tween}\ 80}=2.96\times {10}^{-7}\ {\mathrm{cm}}^2\ {\mathrm{s}}^{-1} \)), which evidences the increase in viscosity of the solutions [6].
The DC polarograms of 1 × 10−3 mol ∙ dm−3 Bi(III) in 2 mol ∙ dm−3 chlorates(VII) ( ) in the presence 1 × 10−3 mol ∙ dm−3 6-thioguanine (
) in the presence 1 × 10−3 mol ∙ dm−3 6-thioguanine ( ) (
) ( ) and 1 × 10−3 mol ∙ dm−3 6-thioguanine with 10−5 mol ∙ dm−3 Tween 80: (
) and 1 × 10−3 mol ∙ dm−3 6-thioguanine with 10−5 mol ∙ dm−3 Tween 80: ( ) 5, (
) 5, ( ) 10, (
) 10, ( ) 20, and (
) 20, and ( ) 30 (a), and with, 10−5 mol ∙ dm−3 Triton X-100: (
) 30 (a), and with, 10−5 mol ∙ dm−3 Triton X-100: ( ) 5, (
) 5, ( ) 10, (
) 10, ( ) 15, (
) 15, ( ) 20, and (
) 20, and ( ) 25 (b). Operational parameter: scan rate 2 mV s−1
) 25 (b). Operational parameter: scan rate 2 mV s−1
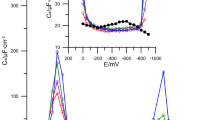
The effect of the studied substances on Bi(III) ion electroreduction can be clearly seen on the electrochemical impedance spectroscopy (Fig. 6a and b). The decreased values of the charge-transfer resistance in the presence of 6-thioguanine (determined from the EIS, where data were collected at 26 frequencies in the range from 200 to 50,000 Hz within the faradaic potential region with 10-mV intervals) indicate that this compound exerts a catalytic effect on Bi(III) electroreduction.
Impedance diagrams measured at \( {E}_f^0 \) for electroreduction of 1 × 10−3 mol ∙ dm−3 Bi(III) in 2 mol ∙ dm−3 chlorates(VII) ( ) in the presence of 1 × 10−3 mol ∙ dm−3 6-thioguanine (
) in the presence of 1 × 10−3 mol ∙ dm−3 6-thioguanine ( ) (
) ( ) and 1 × 10−3 mol ∙ dm−3 6-thioguanine with 10−5 mol ∙ dm−3 Tween 80: (
) and 1 × 10−3 mol ∙ dm−3 6-thioguanine with 10−5 mol ∙ dm−3 Tween 80: ( ) 5, (
) 5, ( ) 10, and (
) 10, and ( ) 20 (a), and with 10−5 mol ∙ dm−3 Triton X-100: (
) 20 (a), and with 10−5 mol ∙ dm−3 Triton X-100: ( ) 5, (
) 5, ( ) 10, and (
) 10, and ( ) 15 (b)
) 15 (b)
The addition of Tween 80 and Triton X-100 causes the increase in the values of the charge-transfer resistance (Fig. 6a and b). Higher and higher values of the charge-transfer resistance are due to a greater and greater difficulty of Bi(III) ion depolarization on the mercury surface and are probably caused by the effect of mixed adsorption layer on the kinetics of the electrode process [10].
Kinetic Parameters
The previous studies dealt with the qualitative estimation of the effect of both 6TG and studied surfactants on the kinetics of Bi(III) ion electroreduction. However, the quantitative criterion of kinetics of Bi(III) ion reduction in the presence of studied substances is obtained from the analysis of kinetic parameters such as cathodic transition coefficient (α) and standard rate constant (ks). The values of transfer coefficient and standard rate constants were obtained from the curves of cyclic voltammetry.
The transfer coefficient (αnα) of electroreduction reaction of Bi(III) ions in the studied systems was determined using the following equation [9]:
The transfer coefficient values depended on the polarization rate; therefore, the extrapolation was conducted out at such polarization rates at which αnα acquires constant values.
For the quasi-reversible processes, the ks values were determined using the method elaborated by Nicholson [9] according to the following equation:
The function Ψ was determined from the product of electron number exchanged in the electrode process (n) and the difference between the potentials of anodic and cathodic peaks (Epa − Epc), and its dependence on n(Epa − Epc) was tabled [16].
For the irreversible processes, the ks values, which are dependent on the kinetic parameters, are described by the following equation [9]:
where
After the addition of 6TG to the basic electrolyte, the values of transfer coefficient increase which indicates an increase in reversibility of Bi(III) ion electroreduction (Table 3). On the other hand, the presence of surface active substances causes a decrease in the parameter α values which results in the changes of Bi(III) ion electroreduction rate towards inhibition. It should be noted that these changes are more significant in the presence of Tween 80. Such changes in the values of the αnα coefficient, associated with the presence of 6-thioguanine in the basic electrolyte solution, according to Petrii and co-workers [17] indicate an asymmetry of intermolecular reorganization and the arbitrary asymmetry factor ν ≪ 1 for zero overvoltage η = 0. During the reduction of Bi-6TG complexes, electrons transferred probably to the antibonding molecular orbital of the complex reactants and the inner-sphere reorganization can be noticeable.
The values of standard rate constants ks confirm the catalytic effect of 6-thioguanine in 2 mol ∙ dm−3 chlorate(VII) solutions. However, the addition of the surfactants to the system shows the tendency towards the ks value reduction. For the same concentration of surfactants, the ks values are always lower in the presence of Tween 80, which proves that it is a more effective inhibitor of Bi(III) ion depolarization on the mercury surface. The studies of adsorption suggest stronger adsorption of Tween 80 compared with Triton X-100 which is explained by the size of surfactant molecules [12, 18]. Tween 80 as a larger molecule favors larger electrode constraint making formation of a suitable number of the mentioned active complexes impossible. It should be also noted that the lack of evident changes of formal potential values (Table 3), with the increasing concentration of studied surfactants, proves that stable complexes are not formed in examined solutions [4].
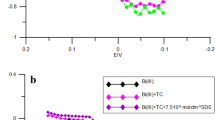
Real rate constants determined from the impedance measurements kf (taking into account the effect of the double layer) [4, 10] of the Bi(III) ion electroreduction in the function of electrode potential indicate that in the presence of the catalyzing substance and the surface active substance, the process of Bi(III) ion electroreduction takes place in stages (Fig. 7a and b). Moreover, the effect of catalyzing substance on the transition of the first electron, which controls the process rate, is greater than that of the transition of other electrons. This is the proof that complexes of Bi(III) ions with the accelerating substance have already been created before the transition of the first electron, which is the slowest stage and determines the rate of the whole process. Active complexes also participate in the transition of successive electrons.
Dependence of the rate constants kf of 1 × 10−3 mol ∙ dm−3 Bi(III) electroreduction ( ) in the presence of 1 × 10−3 mol ∙ dm−3 6-thioguanine (
) in the presence of 1 × 10−3 mol ∙ dm−3 6-thioguanine ( ) (
) ( ) and 1 × 10−3 mol ∙ dm−3 6-thioguanine with 10−5 mol ∙ dm−3 Tween 80: (
) and 1 × 10−3 mol ∙ dm−3 6-thioguanine with 10−5 mol ∙ dm−3 Tween 80: ( ) 5, (
) 5, ( ) 10, and (■) 80 (a), and with 10−5 mol ∙ dm−3 Triton X-100: (
) 10, and (■) 80 (a), and with 10−5 mol ∙ dm−3 Triton X-100: ( ) 5, (
) 5, ( ) 10, (
) 10, ( ), and 25 (b) on the electrode potential
), and 25 (b) on the electrode potential
It is worth mentioning that the Bi-6-thioguanine type complex plays a key role as 6-thioguanine dominates in reaching the adsorption equilibria of the studied mixtures, and what is more, it adsorbs specifically on the electrode surface [18], whereas Tween 80 and Triton X-100 adsorb physically [6, 19,20,21]. With the increasing concentration of surfactants in the electrolyte solution in the presence 1 × 10−3 mol ∙ dm−3 6-thioguanine, a decrease in reversibility of the electrode process is observed.
It should be also emphasized that the stages of dehydration and formation of active complexes in the multi-step Bi(III) ion electroreduction are much faster than those of electron transfer [22, 23]. Therefore, they cannot interfere with each other. The active complexes of Bi(III) ions with catalytic substances are formed when the hydration sphere of Bi(III) ions is partially degraded, and thus, they are located near the outer Helmholtz plane. The studies indicate variety in the structure of these complexes but in the Bi-H2O-6TG combination.
This makes impossible to detect formation of active mixed complexes of Bi-6-thioguanine-surfactants on the electrode surface.
Most probably, the molecules of surfactants start to block the electrode surface pushing out the earlier formed Bi-6TG active complexes from the adsorption layer. A similar situation was observed in the case of 6-mercaptopurine and the surface active substances [12].
Conclusions
The conducted experiments demonstrated the considerable influence of mixed adsorption layers of 6-thioguanine-Tween 80 as well as 6-thioguanine-Triton X-100 on the kinetics and mechanism of the multi-step process of Bi(III) electroreduction in chlorates(VII):
-
6-Thioguanine catalyzes the Bi(III) ion electroreduction process in 2 mol ∙ dm−3 chlorates(VII),
-
The forming mixed adsorption layers reduce the dynamics of Bi(III) ion electroreduction towards the significant inhibition,
-
The nature of lnkf = f(E) changes (non-linearity and change of curve inclination with the change of potential and concentration of the added substances) points out to the multi-stage process of Bi(III) ion electroreduction in 2 mol ∙ dm−3 chlorates(VII),
-
The mechanism of catalytic activity of 6-thioguanine is associated with the formation of complexes on the electrode surface under the specific conditions,
-
The effects of both 6TG and suitable surfactant mixture on the first electron transfer are much greater than those on the transition of other electrons,
-
The composition of active complexes, after subsequent electron transfers, is changed according to the Markus theory [1], which assumes a change in the solvation shell of the ion after a partial loss of charge,
-
The Bi-6TG type complex plays the main role in the Bi-6-thioguanine-Tween 80 or Bi-6-thioguanine-Triton X-100 systems, as 6-thioguanine dominates in the formation of adsorption equilibria in the studied mixtures,
-
The Bi(III) ion electroreduction process becomes slower in the presence of mixed adsorption layers the surfactant molecules probably in the form of hemimicelles start blocking the electrode surface.
References
G. Dalmata, Electroanalysis 17(9), 789–793 (2005)
J. Nieszporek, D. Gugała, D. Sieńko, J. Szaran, J. Saba, Electrochim Acta 51(11), 2278–2281 (2006)
A. Nosal–Wiercińska, Cent Eur J Chem 8, 1–11 (2010)
A. Nosal–Wiercińska, Electroanalysis 26, 1013–1023 (2014)
M. Grochowski, A. Nosal–Wiercińska, J Electroanal Chem 788, 198–202 (2017)
W. Kaliszczak, A.N.–. Wiercińska, J Electroanal Chem 828, 108–115 (2018)
A. Kowalska, Farm Przegl Nauk 5, 15–20 (2009)
D. Zochowska, J. Zegarska, E. Hryniewiecka, E. Samborowska, R. Jazwiec, W. Tszyrsznic, A. Borowiec, M. Dadlez, L. Paczek, Transplant Proc 48(5), 1836–1839 (2016)
A. Nosal–Wiercińska, Electrochim Acta 55, 5917–5921 (2010)
J. Nieszporek, K. Nieszporek, Bull Chem Soc Jpn 91(2), 201–210 (2018)
J. Nieszporek, D. Gugała, D. Sieńko, J. Jankowska, J. Saba, Pol J Chem 78, 1087–1101 (2004)
A. Nosal-Wiercińska, W. Kaliszczak, M. Grochowski, M. Wiśniewska, T. Klepka, J Mol Liq 253, 143–148 (2018)
O. Ikeda, K. Watanabe, Y. Taniguchi, H. Tamura, Bull Chem Soc Jpn 57(12), 3363–3367 (1984)
R.R. Nazmutdinov, W. Schmickler, A.M. Kuznetsov, Chem Phys 310(1-3), 257–268 (2005)
R.R. Nazmutdinov, T.T. Zinkicheva, G.A. Tsirlina, Z.V. Kuz’minova, Electrochim Acta 50(25-26), 4888–4896 (2005)
Z. Galus, Electroanalytical methods of determination of physicochemical constants”, (in Polish) (PWN, Warsaw, 1979)
O.A. Petrii, R.R. Nazmutdinov, M.D. Bronshtein, G.A. Tsirlina, Electrochim Acta 52(11), 3493–3504 (2007)
A. Nosal-Wiercińska, W. Kaliszczak, A. Drapsa, M. Grochowski, M. Wiśniewska, T. Klepka, Adsorption 25(3), 251–256 (2018). https://doi.org/10.1007/s10450-018-9997-3
K. Szymczyk, A. Taraba, Chem Phys 483-484, 96–102 (2017)
M. Wiśniewska, K. Terpiłowski, S. Chibowski, T. Urban, V.I. Zarko, V.M. Gunko, Powder Technol 233, 190–200 (2013)
T. Klepka, H. Dębski, H. Rydarowski, Polymers 54, 668–667 (2009)
M.T.M. Koper, W. Schmickler, A theory for amalgam forming electrode reactions. J Electroanal Chem 450(1), 83–94 (1998)
W. Schmickler, Electron and transfer reactions on metal electrodes. Electrochim Acta 41(14), 2329–2338 (1996)
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Kaliszczak, W., Nosal-Wiercińska, A. Influence of Mixed 6-Thioguanine-Nonionic Surfactant Adsorption Layers on Kinetics and Mechanism of Bi(III) Ion Electroreduction. Electrocatalysis 10, 621–627 (2019). https://doi.org/10.1007/s12678-019-00548-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12678-019-00548-z