Abstract
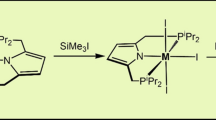
A series of ruthenium [NNN]- or [NCN]-type complexes (3–7) bearing PPh3 ancillary ligands have been synthesized from pyridine- or phenylene-bridged bis(triazoles) 1 and 2. In the case of [NNN]-pincer complex 3, an unusual and unexpected cis-orientation adopted by two sterically demanding PPh3 ligands was observed, and such configuration proved to be unchanged in solution for a long time. By contrast and as expected, the two phosphines are found to be trans to each other in the case of [NCN]-type pincer complex 4, but an oxidation of RuII center to RuIII occurred. Complex cis-3 underwent ligand exchanges leading to the formations of diphosphine derivatives 5 and 6. As a representative, cis-3 was treated with the base in isopropanol affording a mixture of Ru–hydrido complexes with various phosphine binding modes, one of which (trans-7) bearing two trans-standing phosphines has been successfully isolated and fully characterized. The catalytic performances of all newly synthesized Ru complexes have been examined and compared in transfer hydrogenations of ketones and enones, in which mono-phosphine complexes proved to be significantly superior to their diphosphine counterparts. The catalytic process proved to involve Ru–H key intermediates, but the trans-oriented Ru–H species is unlikely to be the main catalytic contributor. In particular, the best performer cis-3 exhibits high chemoselectivity in certain cases catalyzing α,β-unsaturated ketones, whose behavior is quite different compared to most precedents.









Similar content being viewed by others

Notes
References
Byrne JP, Kitchen JA, Gunnlaugsson T (2014) Chem Soc Rev 43:5302
Haldón E, Nicasio MC, Pérez PJ (2015) Org Biomol Chem 13:9528
Liang L, Astruc D (2011) Coord Chem Rev 255:2933
Hein JE, Fokin VV (2010) Chem Soc Rev 39:1302
Meldal M, Tornøe CW (2008) Chem Rev 108:2952
Kolb HC, Finn MG, Sharpless KB (2004) Angew Chem Int Ed 2001:40
Schulze B, Friebe C, Hager MD, Winter A, Hoogenboom R, Görls H, Schubert US (2009) Dalton Trans 5:787
Yang W, Zhong Y (2013) Chin J Chem 31:329
Byrne JP, Kitchen JA, Kotova O, Leigh V, Bell AP, Boland JJ, Albrecht M, Gunnlaugsson T (2014) Dalton Trans 43:196
Gunanathan C, Milstein D (2014) Chem Rev 114:12024
Younus HA, Su W, Ahmad N, Chen S, Verpoort F (2015) Adv Synth Catal 357:283
Younus HA, Ahmad N, Su W, Verpoort F (2014) Coord Chem Rev 276:112
Freeman GR, Williams JAG (2013) Top Organomet Chem 40:89
Deng H, Yu Z, Dong J, Wu S (2005) Organometallics 24:4110
Wang L, Liu T (2018) Chin J Catal 39:327
Wang Q, Chai H, Yu Z (2017) Organometallics 36:3638
Chai H, Liu T, Yu Z (2017) Organometallics 36:4136
Chai H, Wang Q, Liu T, Yu Z (2016) Dalton Trans 45:17843
Wang Q, Wu K, Yu Z (2016) Organometallics 35:1251
Chai H, Liu T, Wang Q, Yu Z (2015) Organometallics 34:5278
Menéndez-Pedregal E, Vaquero M, Lastra E, Gamasa P, Pizzano A (2015) Chem Eur J 21:549
Li K, Niu J-L, Yang M-Z, Li Z, Wu L-Y, Hao X-Q, Song M-P (2015) Organometallics 34:1170
Paul B, Chakrabarti K, Kundu S (2016) Dalton Trans 45:11162
Shi J, Hu B, Chen X, Shang S, Deng D, Sun Y, Shi W, Yang X, Chen D (2017) ACS Omega 2:3406
Toda T, Saitoh K, Yoshinari A, Ikariya T, Kuwata S (2017) Organometallics 36:1188
Melle P, Manoharan Y, Albrecht M (2018) Inorg Chem 57:11761
Shi J, Hu B, Gong D, Shang S, Hou G, Chen D (2016) Dalton Trans 45:4828
Karthikeyan T, Sankararaman S (2009) Tetrahedron Lett 50:5834
Fabbrizzi P, Cicchi S, Brandi A, Sperotto E, van Koten G (2009) Eur J Org Chem 31:5423
Crowley JD, Bandeen PH, Hanton LR (2010) Polyhedron 29:70
Wang H, Zhang B, Yan X, Guo S (2018) Dalton Trans 47:528
Vicente J, Arcas A, Bautista D, Jones PG (1997) Organometallics 16:2127
Clapham SE, Hadzovic A, Morris RH (2004) Coord Chem Rev 248:2201
Bampos N, Field LD, Messerle BA (1993) Organometallics 12:2529
Wang Q, Chai H, Yu Z (2018) Organometallics 37:584
Du W, Wu P, Wang Q, Yu Z (2013) Organometallics 32:3083
Du W, Wang L, Wu P, Yu Z (2012) Chem Eur J 18:11550
Ye W, Zhao M, Du W, Jiang Q, Wu K, Wu P, Yu Z (2011) Chem Eur J 17:4737
Wang D, Astruc D (2015) Chem Rev 115:6621
Bartoszewicz A, Ahlsten N, Martín-Matute B (2013) Chem Eur J 19:7274
Li Y-Y, Yu S-L, Shen W-Y, Gao J-X (2015) Acc Chem Res 48:2587
Alonso F, Riente P, Yus M (2011) Acc Chem Res 44:379
Morris RH (2009) Chem Soc Rev 38:2282
Farrar-Tobar RA, Tin S, de Vries JG (2018) Organometallics for Green Catalysis. Topics Organomet Chem 63:193
Farrar-Tobar RA, Wei Z, Jiao H, Hinze S, de Vries JG (2018) Chem Eur J 24:2725
Melle P, Albrecht M (2019) Chimia 73:299
Horn S, Gandolfi C, Albrecht M (2011) Eur J Inorg Chem 18:2863
Liu T, Chai H, Wang L, Yu Z (2017) Organometallics 36:2914
Fulmer GR, Miller AJM, Sherden NH, Gottlieb HE, Nudelman A, Stoltz BM, Bercaw JE, Goldberg KI (2010) Organometallics 29:2176
Dolomanov OV, Bourhis LJ, Gildea RJ, Howard JAK, Puschmann H (2009) J Appl Cryst 42:339
Acknowledgements
The authors thank “General Project of Scientific Research Program of Beijing Education Commission” (Grant No. KM201810028007), National Natural Science Foundation of China (Grant No. 21502122) and Beijing Natural Science Foundation (Grant No. 2192012) for financial support. The author Dr. Shuai Guo also highly appreciates the support from Yenching Young Scholar Cultivation Program of Capital Normal University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, B., Wang, H., Yan, X. et al. Ruthenium [NNN] and [NCN]-type pincer complexes with phosphine coligands: synthesis, structures and catalytic applications. Transit Met Chem 45, 99–110 (2020). https://doi.org/10.1007/s11243-019-00362-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-019-00362-y