Abstract
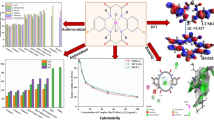
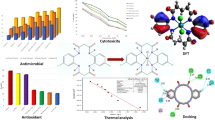
Mixed ligand Ru(II) phenanthroline complexes of the type [Ru(1,10-phen)2Flq]ClO4 (RPFlq-1-3) and “piano-stool”-type Ru(II) arene complexes [Ru(η6-p-cymene)Cl(Flq)] (RAFlq-1-3), where Flq = fluoroquinolone, have been synthesized, characterized and studied for their anticancer potential. DFT calculations were in line with the proposed structures, wherein the fluoroquinolones are coordinated to the metal through the ring carbonyl and one of the carboxylic oxygen atoms in a bidentate fashion. Binding efficacies of the synthesized complexes with bovine serum albumin (BSA) and CT-DNA were studied spectroscopically, and it has been established that the arene complexes, though have moderate binding propensities to CT-DNA (Kb = 0.8–1.7 × 103 M−1), have 102–103-fold better binding efficacies toward BSA (Ka = 3.2 × 105–2.1 × 106 M−1) due to the presence of the hydrophobic arene moiety. These results further prompted a study in their in vitro cytotoxicity assay on A-549 non-small cell lung cancer and MCF7 breast cancer cell lines. Furthermore, gene expression studies on BAX and BCL-2 genes and FACS analysis confirmed apoptosis as the mode of cell death.











Similar content being viewed by others
References
Allardyce CS, Dyson PJ (2001) Platinum Metals Rev. 45:62
Lentzen O, Moucheron C, Kirsch-De Mesmaeker A (2005) Metallotherapeutic drugs & metal-based diagnostic agents. Wiley, West Sussex, pp 359–378
Schluga P (2006) Dalton Trans 14:1796
Pongratz M, Schluga P, Jakupec M, Arion VB, Hartinger C, Allmaier G, Keppler BK (2004) J Anal At Spectrom 19:46
Zanzi I, Srivastava SC, Meinken GE, Robeson W, Mausner LF, Fairchild RG, Margouleff D (1989) Nucl Med Biol 16:397
Srivastava SC (1996) Semin Nucl Med 26:119
Zeng L, Gupta P, Chen Y, Wang E, Ji L, Chao H, Zhe-Sheng C (2017) Chem Soc Rev 46:5771
Barcelo M, Garcia A, Terroon A, Molins E, Prieto MJ, Moreno V, Martinez J, Llado V, Lopez I, Gutierrez A, Escriba PV (2007) J Inorg Biochem 101:649
Hartinger CG, Dyson PJ (2009) Chem Soc Rev 38:391
Y.Yaw Kai, Chem. Comm. 2005, 38, 4764
Soni K (2012) Indo Glob J Pharm Sci 2:43
Ronald AR, Low DE (2003) Fluoroquinolone antibiotics. Birkhäuser, Basel, p 250
Kwok Y, Sun D, Clement JJ, Hurley LH (1999) Anti-Cancer Drug Des 14:443
Yu H, Kwok Y, Hurley LH, Kerwin SM (2000) Biochemistry 39:10236
Rinky S, Ravirajsinh NJ, Menaka CT, Ranjitsinh VD, Debjani C (2012) Transit Met Chem 37:541
Ramadevi P, Rinky S, Sarmita SJ, Ranjitsinh VD, Debjani C (2017) J Organomet Chem 833:80
Ramadevi P, Rinky S, Sarmita SJ, Ranjitsinh VD, Debjani C (2015) J Photochem Photobio AChem 305:1
Sullivan BP, Salmon DJ, Meyer TJ (1978) Inorg Chem 17:3334
Bennett MA, Smith AK (1974) J Chem Soc Dalton Trans (2):233
Bennett MA, Huang TN, Matheson TW, Smith AK (1983) Inorg Synth 21:74
Neese F (2012) Wiley Interdiscip Rev Comput Mol Sci 2:73
Perdew JP (1986) Phys Rev B 33:8822
Becke AD (1988) Phys Rev A 38:3098
Becke AD (1988) Phys Rev A 38:3098
Weigend F, Ahlrichs R (2005) Phys Chem Chem Phys 7:3297
Weigend F (2006) Phys Chem Chem Phys 8:1057
Andrae D, Haeussermann U, Dolg M, Stoll H, Preuss H (1990) Theor Chim Acta 77:123
Vahtras O, Almlöf J, Feyereisen MW (1993) Chem Phys Lett 213:514
Cossi M, Rega N, Scalmani G, Barone V (2003) J Comput Chem 24:669
Hanwell MD, Curtis DE, Lonie DC, Vandermeersch T, Zurek E, Hutchison GR (2012) J Cheminform 4:17
Patel MN, Patel CR, Joshi HN, Thakor KP (2014) Spectrochim Acta, Part A 127:261
Lakowicz JR (1999) Principles of fluorescence spectroscopy, 2nd edn. Plenum Press, New York
Mosmann T (1983) J Immunol Methods 65:55
Deacon GB, Phillips R (1980) J Coord Chem Rev 33:227
Turel I (2002) Coord Chem Rev 232(1–2):27
Ya-Wen T, Yun-Fan C, Yong-Jie L, Kuan-Hung C, Lin C-H, Jui-Hsien H (2018) Molecules 23:159
Adebayo AA, Ajibade PA (2016) J Chem 2016:15, Article ID 3672062
Son G, Yeo J, Kim M, Kim S, Holmen A, Akerman B, Norden B (1998) J Am Chem Soc 120:6451
Pyle AM, Rehmann JP, Meshoyrer R, Kumar CV, Turro NJ, Barton JK (1989) J Am Chem Soc 111:3051
Zhao G, Lin H, Zhu S, Sun H, Chen Y (1998) J Inorg Biochem 70:219
Dhar S, Nethaji M, Chakravarty AR (2005) J Inorg Biochem 99:805
Pasternack RF, Cacca M, Keogh B, Stephenson TA, Williams AP, Gibbs FJ (1991) J Am Chem Soc 113:6835
Lakowicz JR, Weber G (1973) Biochemistry 12:4161
Satyanarayana S, Dabrowiak JC, Chaires JB (1993) Biochemistry 32:2573
Kelly JM, Tossi AB, McConnell DJ (1985) Nucleic Acids Res 13:6017
Suh D, Chaires JB (1995) Bioorg Med Chem 3:723
Wang Y, Zhang H, Zhang G, Tao W, Tang S (2007) J Luminescence 126:211
Ahmad B, Parveen S, Khan RH (2006) Biomacromolecules 7:1350
Mishra B, Barik A, Priyadarsini KI, Mohan H (2005) J Chem Sci 117:641
Petra H, Bock K, Atil B, Hoda MA, Korner W, Bartel C, Jungwirth U, Keppler BK, Michael M, Berger W, Gunda K (2010) J Biol Inorg Chem 15:737
Tan C, Hu S, Liu J, Liangnian J (2011) Eur J Med Chem 46:1555
Wee HA, Elisa D, Claudine S, Rosario S, Lucienne J, Dyson PJ (2006) Inorg Chem 45:9006
Acknowledgements
The authors gratefully acknowledge the University Grants Commission RFSMS-BSR fellowship [UGC No. F.4-1/2006 (BSR)/5-68/2007 (BSR)], New Delhi, for financial assistance. The authors are thankful to the Head, Department of Chemistry and Department of Zoology, Faculty of Science, The Maharaja Sayajirao University of Baroda, for providing us with the necessary laboratory facilities and required instrumentation facilities to carry out the research work. The authors thank DBT-MSUB-ILSPARE project, Dr. Vikram Sarabhai Science Block, M. S. University of Baroda, for providing the ESI MS analysis of the complexes and FACS analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pulipaka, R., Dash, S.R., Khanvilkar, P. et al. Design, synthesis and in vitro bioactivity of mixed ligand Ru(II) complexes bearing the fluoroquinolone antibacterial agents. Transit Met Chem 44, 721–735 (2019). https://doi.org/10.1007/s11243-019-00341-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-019-00341-3