Abstract
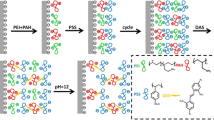
Composite polyelectrolyte pervaporation membranes were fabricated through layer-by-layer self-assembly method. Cationic polyethyleneimine (PEI) and anionic poly(4-styrene sulfonic acid-co-maleic acid) (PSSMA) polyelectrolytes were deposited one after the other on a porous asymmetric modified polyacrylonitrile (mPAN) substrate. Attenuated total reflection–Fourier transform infrared spectral analysis and X-ray photoelectron spectrometry confirmed the deposition of polyelectrolytes on the mPAN support. Field emission scanning electron microscopy depicted that pores in the mPAN support were covered with layers of polyelectrolytes. Atomic force microscopy illustrated that the deposition of polyelectrolytes led to a smooth surface of the membrane. Moreover, the membrane exhibited improved hydrophilicity. We investigated the effect of polyelectrolyte concentrations on the efficiency of dehydrating 90 wt% aqueous alcohol solutions through pervaporation. The results established that bilayer polyelectrolyte membranes fabricated from 0.9 wt% PEI and 0.1 w% PSSMA delivered maximal dehydration efficiency.







Similar content being viewed by others
References
Shao P, Huang RYM (2007) Polymeric membrane pervaporation. J Membr Sci 287(2):162–179. https://doi.org/10.1016/j.memsci.2006.10.043
Sun Y-M, Huang T-L (1995) Pervaporation of ethanol-water mixtures through poly(vinyl alcohol-g-acrylic acid) membranes prepared by use of Fenton's reagent in grafting. J Polym Res 2(1):47–53. https://doi.org/10.1007/bf01493433
Jyoti G, Keshav A, Anandkumar J (2015, 2015) Review on pervaporation: theory, membrane performance, and application to intensification of esterification reaction. J Eng. https://doi.org/10.1155/2015/927068
Ong YK, Shi GM, Le NL, Tang YP, Zuo J, Nunes SP, Chung TS (2016) Recent membrane development for pervaporation processes. Prog Polym Sci 57:1–31. https://doi.org/10.1016/j.progpolymsci.2016.02.003
Cannilla C, Bonura G, Frusteri F (2017) Potential of Pervaporation and vapor separation with water selective membranes for an optimized production of biofuels—a review. Catalysts 7(6):187. https://doi.org/10.3390/catal7060187
Khebudkar R, Nangare D, Wagh M (2017) A review on energy efficient hybrid distillation/Pervaporation process for separation of isopropyl alcohol Azeotrope. Int J Eng Adv Technol 6:141–144. https://doi.org/10.1016/S0376-7388(98)00253-1
Liu H-X, Wang N, Zhao C, Ji S, Li J-R (2017) Membrane materials in the pervaporation separation of aromatic/aliphatic hydrocarbon mixtures—a review. Chin J Chem Eng. https://doi.org/10.1016/j.cjche.2017.03.006
Wang Y-H, Lee K-R, Liaw D-J, Lai J-Y (1998) Permselectivities of aromatic polyamide membranes for aqueous alcohol mixtures in pervaporation. J Polym Res 5(1):31–36. https://doi.org/10.1007/s10965-006-0037-8
de La Cruz MO, Belloni L, Delsanti M, Dalbiez J, Spalla O, Drifford M (1995) Precipitation of highly charged polyelectrolyte solutions in the presence of multivalent salts. J Chem Phys 103(13):5781–5791. https://doi.org/10.1063/1.470459
Barrat JL, Joanny JF (1996) Theory of polyelectrolyte solutions. Adv Chem Phys 94:1–66. https://doi.org/10.1002/9780470141533.ch1
Thünemann AF, Müller M, Dautzenberg H, Joanny J-F, Löwen H (2004) Polyelectrolyte complexes. In: Polyelectrolytes with defined molecular architecture II. Advances in Polymer Science, vol 166. In: Schmidt M. (eds) edn. Springer, Berlin, Heidelberg, pp 113-171. https://doi.org/10.1007/b11350
Zhao Q, An QFF, Ji YL, Qian JW, Gao CJ (2011) Polyelectrolyte complex membranes for pervaporation, nanofiltration and fuel cell applications. J Membr Sci 379(1–2):19–45. https://doi.org/10.1016/j.memsci.2011.06.016
Saren Q, Qiu CQ, Tang CY (2011) Synthesis and characterization of novel forward osmosis membranes based on layer-by-layer assembly. Environ Sci Technol 45(12):5201–5208. https://doi.org/10.1021/es200115w
Zhao Q, Qian JW, An QF, Sun ZW (2010) Layer-by-layer self-assembly of polyelectrolyte complexes and their multilayer films for pervaporation dehydration of isopropanol. J Membr Sci 346(2):335–343. https://doi.org/10.1016/j.memsci.2009.09.055
Joseph N, Ahmadiannamini P, Hoogenboom R, Vankelecom IFJ (2014) Layer-by-layer preparation of polyelectrolyte multilayer membranes for separation. Polym Chem 5(6):1817–1831. https://doi.org/10.1039/c3py01262j
Mortimer DA (1991) Synthetic polyelectrolytes—a review. Polym Int 25(1):29–41. https://doi.org/10.1002/pi.4990250107
Shieh J-J, Huang RYM (1997) Pervaporation with chitosan membranes II. Blend membranes of chitosan and polyacrylic acid and comparison of homogeneous and composite membrane based on polyelectrolyte complexes of chitosan and polyacrylic acid for the separation of ethanol-water mixtures. J Membr Sci 127(2):185–202. https://doi.org/10.1016/S0376-7388(96)00279-7
Toutianoush A, Krasemann L, Tieke B (2002) Polyelectrolyte multilayer membranes for pervaporation separation of alcohol/water mixtures. Colloids Surf A Physicochem Eng Asp 198:881–889. https://doi.org/10.1016/S0927-7757(01)01015-9
Deng HY, Xu YY, Zhu BK, Wei XZ, Liu F, Cui ZY (2008) Polyelectrolyte membranes prepared by dynamic self-assembly of poly (4-styrenesulfonic acid-co-maleic acid) sodium salt (PSSMA) for nanofiltration (I). J Membr Sci 323(1):125–133. https://doi.org/10.1016/j.memsci.2008.06.028
Guo H, Chen M, Liu Q, Wang Z, Cui S, Zhang G (2015) LbL assembly of sulfonated cyclohexanone–formaldehyde condensation polymer and poly(ethyleneimine) towards rejection of both cationic ions and dyes. Desalination 365:108–116. https://doi.org/10.1016/j.desal.2015.01.021
Tjipto E, Quinn JF, Caruso F (2005) Assembly of multilayer films from polyelectrolytes containing weak and strong acid moieties. Langmuir 21(19):8785–8792. https://doi.org/10.1021/la051197h
Gao J, Ran X, Shi C, Cheng H, Cheng T, Su Y (2013) One-step solvothermal synthesis of highly water-soluble, negatively charged superparamagnetic Fe3O4 colloidal nanocrystal clusters. Nanoscale 5(15):7026–7033. https://doi.org/10.1039/c3nr00931a
Lv Y, Yang HC, Liang HQ, Wan LS, Xu ZK (2015) Nanofiltration membranes via co-deposition of polydopamine/polyethylenimine followed by cross-linking. J Membr Sci 476:50–58. https://doi.org/10.1016/j.memsci.2014.11.024
Nan Q, Li P, Cao B (2016) Fabrication of positively charged nanofiltration membrane via the layer-by-layer assembly of graphene oxide and polyethylenimine for desalination. Appl Surf Sci 387:521–528. https://doi.org/10.1016/j.apsusc.2016.06.150
Ishigami T, Amano K, Fujii A, Ohmukai Y, Kamio E, Maruyama T, Matsuyama H (2012) Fouling reduction of reverse osmosis membrane by surface modification via layer-by-layer assembly. Sep Purif Technol 99:1–7. https://doi.org/10.1016/j.seppur.2012.08.002
Saqib J, Aljundi IH (2016) Membrane fouling and modification using surface treatment and layer-by-layer assembly of polyelectrolytes: state-of-the-art review. J Water Process Eng 11:68–87. https://doi.org/10.1016/j.jwpe.2016.03.009
Kononova SV, Volod'ko AV, Petrova VA, Kruchinina EV, Baklagina YG, Chusovitin EA, Skorik YA (2018) Pervaporation multilayer membranes based on a polyelectrolyte complex of lambda-carrageenan and chitosan. Carbohydr Polym 181:86–92. https://doi.org/10.1016/j.carbpol.2017.10.050
Chen Y, Xiangli F, Jin W, Xu N (2007) Organic–inorganic composite pervaporation membranes prepared by self-assembly of polyelectrolyte multilayers on macroporous ceramic supports. J Membr Sci 302(1–2):78–86. https://doi.org/10.1016/j.memsci.2007.06.019
Krasemann L, Tieke B (1998) Ultrathin self-assembled polyelectrolyte membranes for pervaporation. J Membr Sci 150(1):23–30. https://doi.org/10.1016/S0376-7388(98)00212-9
Zhang Y, Rhim JW, Feng XS (2013) Improving the stability of layer-by-layer self-assembled membranes for dehydration of alcohol and diol. J Membr Sci 444:22–31. https://doi.org/10.1016/j.memsci.2013.05.011
Zhang G, Gu W, Ji S, Liu Z, Peng Y, Wang Z (2006) Preparation of polyelectrolyte multilayer membranes by dynamic layer-by-layer process for pervaporation separation of alcohol/water mixtures. J Membr Sci 280(1–2):727–733. https://doi.org/10.1016/j.memsci.2006.02.031
Zhao J, Zhu YW, Pan FS, He GW, Fang CH, Cao KT, Xing RS, Jiang ZY (2015) Fabricating graphene oxide-based ultrathin hybrid membrane for pervaporation dehydration via layer-by-layer self-assembly driven by multiple interactions. J Membr Sci 487:162–172. https://doi.org/10.1016/j.memsci.2015.03.073
Liu G, Jiang Z, Li C, Hou L, Chen C, Yang H, Pan F, Wu H, Zhang P, Cao X (2019) Layer-by-layer self-assembled nanocomposite membranes via bio-inspired mineralization for pervaporation dehydration. J Membr Sci 570-571:44–52. https://doi.org/10.1016/j.memsci.2018.09.067
Acknowledgements
The authors would like to acknowledge the Ministry of Science and Technology of Taiwan (MOST 106-2221-E-197-024 and MOST 107-2221-E-197-014) for financially supporting this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
De Guzman, M.R., Ang, M.B.M.Y., Huang, SH. et al. Layer-by-layer self-assembly of polyethyleneimine and poly(4-styrene sulfonic acid-co-maleic acid) forming composite polyelectrolyte membranes for pervaporation of aqueous alcohol solutions. J Polym Res 26, 286 (2019). https://doi.org/10.1007/s10965-019-1977-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-019-1977-0