Abstract
A series of hydrazide compounds were synthesized and employed as nucleating agents for isotactic polypropylene (iPP). The effect of different structures of hydrazide compounds on the crystallization and melting behaviour of iPP was studied by differential scanning calorimetry (DSC). The results demonstrate that almost all nucleating agents are α-nucleating agents, some of which are highly efficient nucleating agents. In the case of the same substituent, the greater the number of hydrazide groups, the better the nucleation effect. The crystallization peak temperature (Tc) of iPP nucleated with dihydrazide is 3–4 °C higher than that of monohydrazide. When the substituent of the dihydrazide compound is hydrogen, ethyl and nonyl, their nucleating ability is relatively weak, but in the state of phenyl and cyclohexyl substitution, the Tc of iPP nucleated with dihydrazide nucleating agent is 8.5 °C than pure iPP. When the intermediate group of the dihydrazide compound is a long-chain alkyl, a phenyl, a cyclohexyl, and a naphthyl group, the phenyl group and the cyclohexyl group still exhibit an excellent effect, the Tc of nucleated iPP is raised by about 10 °C.
Similar content being viewed by others
Introduction
As one of the most widely used commercial thermoplastics, isotactic polypropylene (iPP) exhibits excellent comprehensive properties, for example, low manufacturing cost, high heat resistance and good mechanical properties. Owing to these advantages, iPP is commonly used in packaging, home appliances and other industrial applications. However, a lot of drawbacks of iPP limit its further application, such as poor transparency, large size of spherulite and slow crystallization [1,2,3,4,5,6,7]. As such, finding an effective approach to improve the grain size and crystallization rate of iPP is a top priority. So far, physical modification and chemical modification have been taken to improve crystallization behavior of iPP. Chemical modification mainly includes copolymerization, grafting, crosslinking, etc., and the modification is achieved by changing the molecular structure of iPP [8,9,10,11]. Physical modification consists of mixing, filling, reinforcement, etc., and additives impart new properties to iPP [12, 13]. Among these modification methods, the addition of a nucleating agent has received extensive attention due to its high efficiency and convenience. In fact, the nucleating agent not only speeds up the crystallization process, increases the density of the nucleus, reduces the spherulite size, and has no effect on the chemical structure of the polymer [14,15,16,17].
iPP is a polymorphic composition that can crystallize in α, β, γ, ε and smectic modifications [18,19,20]. The α-form is the most common and steady crystalline form of these crystal forms and it also has significant thermal stability and rigidity. The α-nucleating agent can induce the nucleation of iPP resin in the α crystal form, while increasing the crystallization rate and impart the product brightness and other functions [21]. To date, α-nucleating agent is the most extensively used nucleating agent in the market. Five types of organic compounds are mainly used as α-nucleating agents: aromatic carboxylate, sorbitol derivative, organic phosphate salt, dehydroabietic and its salt and amide derivative. Although these compounds can utilized as α-nucleating agent, only a few substances, such as 2,2-methylene-bis (4,6-di-tert-butylphenyl) phosphate sodium (NA-11) and NA-21, which the main component is 2,2-methylene-bis (4,6-di-tert-butylphenyl) phosphate aluminum salt, are already on the market [22]. Unfortunately, these two nucleating agents have poor dispersibility and low nucleation efficiency. Other nucleating agents also have similar disadvantages, such as high production costs, discoloration, and so on [23,24,25]. Therefore, finding a highly efficient and excellent α-nucleating agent is still required, not only a rudimentary scientific challenge, but also important for industrial applications.
In recent years, some hydrazide compounds have received extensive attention due to their high efficiency nucleation in poly(L-lactic acid) [26,27,28,29,30]. Kawamoto observed that octamethlenedicarboxylic dibenzoylhyhydrazide and decamethylenedicarboxylic dibenzoylhydrazide showed remarkable nucleating ability in poly(L-lactic acid) [27]. Subsequently, Cai found that N, N′-Bis(benzoyl) suberic acid dihydrazide can increase the number of spherulites of poly (L-lactic acid) and decrease the size of spherulites [28]. Among them, decamethylenedicarboxylic dibenzoylhydrazide (TMC-300) has been applied to poly (L-lactic acid) and commercialized. Zhang et al. found that TMC-300 also has excellent nucleation effect in iPP, which not only reduces the spherulite size and half crystallization time, but also increases flexural modulus and heat distortion temperature [29, 30]. Currently, there are few systematic studies on hydrazide compounds as nucleating agents in iPP. Based on these researches, we synthesized hydrazide compounds with different chemical structures and utilized them as nucleating agent for iPP. In this study, the crystallization and melting behaviors of iPP nucleated with different hydrazide compounds will be studied by the differential scanning calorimetry (DSC), so as to figure out the relationship between the chemical structure of hydrazide compounds and iPP nucleation. Meanwhile, the one step or two step synthesis route of the hydrazide compounds allows the chemical structure to be changed, thereby achieving the purpose of tailoring the nucleating agent for iPP and optimizing its nucleation efficiency [31, 32]. It also provides guidance for the design of iPP nucleating agent molecules in the future.
Experimental
Materials
The iPP sample (MFR = 2.5 g/(10 min), isotactic index = 94.5%) was provided by SINOPEC Baling Co. (China). Antioxidants 168 and 1010 were purchased from Ciba Specialty Chemicals (Switzerland). A series of hydrazide compound nucleating agents are synthesized according to the references and patents [33, 34], and the structures are listed in Table 1.
Preparation of nucleated iPP samples
iPP and hydrazide nucleating agent (0.3 mass% in iPP) blends were prepared by using a SHR 100 high-speed mixer (Zhangjiagang HaiChuan Machinery Co. Ltd., China). The antioxidants (0.1 mass% in iPP) were added to all samples to control oxidation of iPP during processing. The pellets of iPP were produced utilizing a SHJ-20B twin-screw extruder (Nanjing GIANT Machinery Co. Ltd., China). These treated samples were prepared for follow-up DSC analysis.
Differential scanning calorimetry (DSC) measurements
The crystallization and melting behavior of pure iPP and nucleated iPP were analyzed on a TA Q2000 DSC (TA Instruments Co., U.S.A) under the protection of nitrogen, and the indium was used as a standard medium to calibrate the temperature prior to the tests. For all samples, the weight was 4–5 mg, first reached to 210 °C and held for 3 min to ensure complete removal of heat history. Subsequently, all samples were cooled down to 50 °C at 10 °C/min, and reached 210 °C at the heating rate 10 °C/min. The corresponding data were automatically collected by the equipment.
Results and discussion
Effect of the number of hydrazide groups on the crystallization and melting behavior of iPP
The structures of the hydrazide compounds we studied are given in Table 1. In this section, we have studied compounds of benzoyl hydrazide and terephthalic acid dihydrazide substituted with different substituents, including phenyl, cyclohexyl and ethyl. It is worth pointing out that the melting point of the H-substituted benzoyl hydrazide is 108.6 °C, and the Et-substituted benzoyl hydrazide melts when dried at 60 °C under normal pressure, as such, these two compounds are not utilized in this study [35].
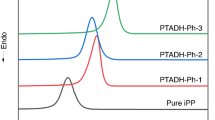
The first DSC cooling scans of pure iPP and nucleated iPP are shown in Fig. 1. From Fig. 1, it is apparent that both PTADH-R and BH-R can increase the crystallization peak temperature (Tc) of iPP. In Fig. 1a, PTADH-Cy and PTADH-Ph had better effects, the Tc of nucleated iPP increased was from 119.9 °C (pure iPP) to 129.5 °C and 130.6 °C, respectively, which showed the strong heterogeneous nucleation ability of nucleating agents. However, other two dihydrazide compounds have less increased in the Tc of iPP, indicating that H, Et-substituted terephthalic acid dihydrazide compounds have comparatively weaker nucleation effects on iPP. Figure 1b show that the Tc of iPP nucleated with BH-Ph and BH-Cy was increased to 126.2 °C and 126.8 °C, respectively. Comparing the two types of nucleating agents, the former has more improvement on the Tc of iPP than latter under the premise of the same substituent. Since monohydrazide compounds do not achieve the desired results, they are not discussed here. Hereinafter, the dihydrazide compounds are selected for comparison with other hydrazide nucleating agents.
The second DSC heating scans of nucleated iPP and pure iPP are presented in Fig. 2. It could be observed that the melting curves of iPP nucleated with PTADH-R and BH-Cy only have one peak at about 162 °C, and the melting peak belongs to the melting of α-phase iPP, suggesting that this compound only can induce α-formed crystal for iPP. However, the melting curve of iPP nucleated with BH-Ph not only observed a melting peak at about 162 °C, but also a melting peak at about 147 °C, which is a typical β melting peak. It indicates that BH-Ph has dual nucleation ability.
Effect of dihydrazide compounds with different side chain groups on crystallization and melting behavior of iPP
In this section, the effect of dihydrazide compounds with different side chain groups on iPP was investigated. In order to avoid errors caused by lack of data, three different types of dihydrazide compound were used for comparison. The main substituents include ethyl, nonyl, cyclohexyl and phenyl. In previous studies, our group found that the compound of sebacic acid dihydrazide has the greatest improvement in the Tc of iPP, so it was chosen for further comparison. Meanwhile, in order to make the result clearer, the other two hydrazide compounds select terephthalic acid dihydrazide and 1, 4-cyclohexanedicarboxylic acid dihydrazide.
Figure 3a–c show the the first DSC cooling scans of iPP nucleated with PTADH-R, SDH-R and CHDADH-R, respectively. Figure 3 shows that these nucleating agents, more or less, increase the Tc of iPP. In Fig. 3a, PTADH-Cy and PTADH-Ph exhibited excellent effects, the Tc of iPP are increased by 10.7 °C and 9.6 °C, respectively. A similar conclusion can be drawn from Fig. 3b, c, the Tc of iPP nucleated with SDH-Cy, SDH-Ph, CHDADH-Cy and CHDADH-Ph increased from 119.9 °C to 129.7 °C, 128.4 °C, 130.1 °C and 129.5 °C, which indicated that Cy, Ph-substituted dihydrazide compounds have excellent nucleation effect of iPP. Compared to the Cy, Ph-substituted dihydrazide compounds, the Tc of the iPP nucleated with other dihydrazides is relatively low. A careful observation of the Fig. 3, it can be seen that the order of nucleation of the five substituents from good to bad was probably Cy > Ph > Et > No > H. In that case, the cyclohexy and phenly-substituted dihydrazide compounds are highly efficient α-nucleating agent for iPP. Accordingly, the cyclohexy and phenyl group were chosen for further investigation.
The second DSC heating scans of iPP nucleated with PTADH-R, SDH-R and CHDADH-R are exhibited in Fig. 4. It is obvious that the introduction of hydrazide nucleating agents also increases the melting peak temperature of iPP. All samples have only one melting peak at about 162 °C, which belongs to the α-crystals. This suggests that these hydrazide compounds are all α-nucleating agents.
Effect of dihydrazide compounds with different central groups on crystallization and melting behavior of iPP
According to the previous studies, the Tc of the iPP nucleated with cyclohexy and phenly-substituted dihydrazide compounds is relatively high. Hence, in this section, the phenyl and cyclohexyl group were selected as substituents, the effect of dihydrazide compounds with different central groups on the crystallization and melting behavior of iPP was studied, and the main central group includes long-chain alkyl groups, phenyl, cyclohexyl and naphthyl.
Figure 5 displays the first DSC cooling scans of pure iPP and iPP nucleated with cyclohexy and phenly-substituted dihydrazide compounds, and it can be observed that all nucleating agents induce the obvious increase of crystallization peak temperature of iPP. Figure 5a shows that when the central group is naphthyl, the Tc of iPP nucleated with dihydrazide is much lower than that of other iPP. The Tc of iPP nucleated with SDH-Ph, CHDADH-Ph and PTADH-Ph are as high as 128.4 °C, 129.5 °C and 129.5 °C, while the the Tc of iPP nucleated with NDADH-Ph only 125.2 °C. The change trend of Fig. 5b is in accordance with Fig. 5a, the Tc of iPP nucleated with SDH-Cy, CHDADH-Cy and PTADH-Cy are all around 130 °C, and NDADH-Cy only increases by 0.7 °C. The order of nucleation of the four central groups from good to bad was phenyl > cyclohexyl > long-chain alkyl > naphthyl.
The second DSC heating scans of iPP nucleated with phenyl and cyclohexyl substituted dihydrazide are displayed in Fig. 6. The results indicated that the addition of these hydrazide compounds lead to increasing in melting peak. It can be seen from the melting peak around 162 °C in Fig. 6, all nucleating agents can only induce α-crystal forms, indicating that these hydrazide compounds are all α-nucleating agents.
Okada proposes that hydrogen bonding interactions directly affect the crystallization of polymers nucleated with nucleating agents [36]. Based on this theory, the researchers found that the dihydrazide nucleating agent can form hydrogen bonds with poly (1, 4-butylene adipate) and poly (L-lactide) to promote crystallization [32, 37]. In order to understand the hydrogen bond formation process and crystallization process between dihydrazide and iPP, taken iPP/PTADH-Ph for instance, a schematic diagram is displayed in Fig. 7. In the initial period of crystallization, iPP long chains are freely distributed around PTADH-Ph (Fig. 7a), subsequently, possible hydrogen bond adsorption occurs on PTADH-Ph (Fig. 7b), the hydrogen bonds are formed between the hydrogen atom of iPP and the -C=O group of PTADH-Ph. The hydrogen bond interaction leads to migration of the iPP molecular chain to PTADH-Ph, allowing nucleation and orderly growth of the iPP on PTADH-Ph (Fig. 7c, d). It can be speculated that the hydrogen bond between the iPP and the nucleating agent promotes the crystallization process of the iPP. Therefore, the nucleation effect of the dihydrazide compounds is better than that of the monohydrazide compounds.
As noted in the reference, the iPP lamellae formed randomly on the surface of nucleating agent in the earlier stages of crystallization. The total crystallization rate of iPP depends on the nucleation process, which in turn is linked to the steric hindrance of the nucleating agent molecules [38, 39]. In Sections 3.2, when phenyl and cyclohexyl groups are used as side chain groups, the Tc of iPP nucleated with dihydrazide compounds is increased by more than 8.5 °C. At the same time, in Section 3.3, when phenyl and cyclohexyl groups are used as intermediate groups, excellent effects are still exhibited, and the Tc of iPP is as high as about 130 °C. This may be due to the smaller steric hindrance of the phenyl and cyclohexyl groups, which provides more growth sites for the epitaxial growth of iPP and promotes crystallization of iPP on nucleating agents. Although these are just a few speculations, they also provide research directions for the mechanism of nucleation of hydrazide nucleating agents. Weak hydrogen bonding and steric hindrance of groups can’t be overlooked.
Conclusions
In summary, the crystallization and melting behavior of iPP nucleated with different hydrazide compounds was studied by DSC analysis. The results demonstrate that the structure of hydrazide compounds plays an important role in the nucleation effect, namely, the number of hydrazide groups, the type of substituents and intermediate groups. First of all, in the case of same substituents, the Tc of iPP nucleated with dihydrazide is 3–4 °C higher than that of monohydrazide, indicating that the nucleation effect of dihydrazide is better than that of monohydrazide. What’s more, when the substituent of the dihydrazide is hydrogen, ethyl and nonyl, their nucleation ability is relatively weak, but in the state of phenyl and cyclohexyl substitution, the Tc of iPP is maintained at above 128.4 °C, which is 8.5 °C higher than pure iPP. This means that the phenyl group and the cyclohexyl group are suitable side chain groups when used as a substituent of the dihydrazide. Finally, when the central group is long-chain alkyl groups, phenyl, cyclohexyl and naphthyl, the phenyl group and the cyclohexyl group still exhibit an excellent effect, and the Tc of the iPP is raised by about 10 °C.
References
Zhao SC, Yu X, Gong HZ, Shi YQ, Zhou S (2015) The crystallization behavior of isotactic polypropylene induced by a novel anti-nucleating agent and its inhibition mechanism of nucleation. Ind Eng Chem Res 54(31):7650–7657
Kristiansen M, Werner M, Tervoort T, Smith P, Blomenhofer M, Schmidt HW (2003) The binary system isotactic polypropylene/bis(3,4-dimethylbenzylidene) sorbitol: phase behavior, nucleation, and optical properties. Macromolecules 36(14):5150–5156
Zhang YF (2008) Comparison of nucleation effects of organic phosphorous and sorbitol derivative nucleating agents in isotactic polypropylene. J Macromol Sci B 47(6):1188–1196
Libster D, Aserin A, Garti N (2010) Advanced nucleating agents for polypropylene. Polym Adv Technol 18(9):685–695
Zhang YF, Hou HH, Guo LH (2018) Effects of cyclic carboxylate nucleating agents on nucleus density and crystallization behavior of isotactic polypropylene. J Therm Anal Calorim 131(2):1483–1490
Yu YS, Xiong BJ, Zeng FXY, Xu RZ, Yang F, Kang J, Xiang M, Li L, Sheng XY, Hao ZH (2018) Influences of compression on the mechanical behavior and electrochemical performances of separators for lithium ion batteries. Ind Eng Chem Res 57(50):17142–17151
Xiong BJ, Chen R, Zeng FXY, Kang J, Men YF (2018) Thermal shrinkage and microscopic shutdown mechanism of polypropylene separator for lithium-ion battery: in-situ ultra-small angle X-ray scattering study. J Membr Sci 545:213–220
Wang X, Tzoganakis C, Rempel GL (1996) Chemical modification of polypropylene with peroxide/pentaerythritol triacrylate by reactive extrusion. J Appl Polym Sci 61(8):1395–1404
John MJ, Anandjiwala RD (2009) Chemical modification of flax reinforced polypropylene composites. Compos Part A-Appl S 40(4):442–448
Ismail SH, Bakar AA (2006) Effects of chemical modification of paper sludge filled polypropylene, (PP)/ethylene propylene diene terpolymer (EPDM) composites. J Reinf Plast Compos 25(1):43–58
Mahlberg R, Paajanen L, Nurmi A, Kivistö A, Koskela K, Rowell RM (2001) Effect of chemical modification of wood on the mechanical and adhesion properties of wood fiber/polypropylene fiber and polypropylene/veneer composites. Holz Roh Werkst 59(5):319–326
Son TW, Lim SK, Chang CM, Kim SS, Cho IS (2010) Physical modification of polypropylene: preparation of fibres dyeable with disperse dyes. Color Technol 115(12):366–369
Son TW, Lim SK, Lee DW, Lee EW (2015) Physical modification of polypropylene. III. Novel morphology of polypropylene and poly (ethylene-co-vinyl alcohol) with epoxy blend fibers. J Appl Polym Sci 73(6):1049–1057
Yu YS, Xu RZ, Chen JY, Kang J, Xiang M, Li YJ, Li L, Sheng XY (2019) Ordered structure effects on β-nucleated isotactic polypropylene/graphene oxide composites with different thermal histories. RSC Adv 9(34):19630–19640
Zhang YF, Chen H, Liu BB, Gu YH, Li XX (2014) Isothermal and non-isothermal crystallization of isotactic polypropylene nucleated with 1,3,5-benzenetricarboxylic acid tris(cyclohexylamide). Thermochim Acta 590:226–231
Zhang YF, Chen H (2014) Effects of nucleating agent 1,3,5-benzenetricarboxylic acid tris(cyclohexylamide) on properties and crystallization behaviors of isotatic polypropylene. Colloid Polym Sci 292(2):493–498
Zhao SC, Cai Z, Xin Z (2008) A highly active novel β-nucleating agent for isotactic polypropylene. Polymer 49(11):2745–2754
Chen L, Yang YD, Xin Z, Qin W, Zhou S, Zhao SC (2019) Increased nucleation efficiency of an in situ-formed β-nucleating agent for impact polypropylene copolymer. J Polym Res 26(10):245
Varga J (1992) Supermolecular structure of isotactic polypropylene. J Mater Sci 27(10):2557–2579
Lotz B (2014) A new ε crystal modification found in stereodefective isotactic polypropylene samples. Macromolecules 47(21):7612–7624
Zheng H, Zeng FXY, Chen ZF, Kang J, Chen JY, Cao Y, Xiang M (2017) Exploring the roles of molecular structure on the β-crystallization of polypropylene random copolymer. J Polym Res 24(12):225
Ghugare SV, Govindaiah P, Avadhani CV (2009) Polypropylene-organoclay nanocomposites containing nucleating agents. Polym Bull 63(6):897–909
Zhao SC, Liu KH, Zhou S, Shi YQ, Xin Z (2017) A novel self-dispersed β nucleating agent for isotactic polypropylene and its unique nucleation behavior and mechanism. Polymer 132:69–78
Abraham F, Ganzleben S, Hanft D, Smith P, Schmidt HW (2010) Synthesis and structure–efficiency relations of 1,3,5-benzenetrisamides as nucleating agents and clarifiers for isotactic poly(propylene). Macromol Chem Phys 211(2):171–181
Ferreira CI, Dal Castel C, Oviedo MAS, Mauler RS (2013) Isothermal and non-isothermal crystallization kinetics of polypropylene/exfoliated graphite nanocomposites. Thermochim Acta 553:40–48
Li CH, Luo SS, Wang JF, Wu H, Guo SY, Zhang X (2017) Conformational regulation and crystalline manipulation of PLLA through a self-assembly nucleator. Biomacromolecules 18(4):1440–1448
Kawamoto N, Sakai A, Horikoshi T, Urushihara T, Tobita E (2010) Physical and mechanical properties of poly(L-lactic acid) nucleated by dibenzoylhydrazide compound. J Appl Polym Sci 103(1):244–250
Cai YH, Yan SF, Yin JB, Fan YQ, Chen XS (2011) Crystallization behavior of biodegradable poly (L-lactic acid) filled with a powerful nucleating agent: N,N′-bis(benzoyl) suberic acid dihydrazide. J Appl Polym Sci 121(3):1408–1416
Zhou PZ, Zhang YF, Lin XF (2019) Crystallization kinetics of isotactic polypropylene nucleated with octamethylenedicarboxylic dibenzoylhydrazide under isothermal and non-isothermal conditions. J Therm Anal Calorim 136(2):749–757
Zhang YF, Zhou PZ, Mao JJ, Liu N (2019) Influences of octamethylenedicarboxylic dibenzoylhydrazide on crystallization, melting behaviors, and properties of isotactic polypropylene. Polym Bull 76(4):1685–1696
Wilsens CHRM, Hawke LGD, Troisi EM, Hermida-Merino D, de KortG LN, Saralidze K, Peters GWM, Rastogi S (2018) Effect of self-assembly of oxalamide based organic compounds on melt behavior, nucleation, and crystallization of isotactic polypropylene. Macromolecules 51(13):4882–4892
Yang Y, Liang R, Chen Y, Zhang C, Zhang R, Wang X, Kong R, Chen Q (2017) Using a self-assemblable nucleating agent to tailor crystallization behavior, crystal morphology, polymorphic crystalline structure, and biodegradability of poly(1,4-butylene adipate). Ind Eng Chem Res 56(28):7910–7919
Kawamoto N, Sakai A, Horikoshi T, Urushihara T, Tobita E (2007) Nucleating agent for poly(L-lactic acid)—an optimization of chemical structure of hydrazide compound for advanced nucleation ability. J Appl Polym Sci 103(1):198–203
Wang KZ, Li XG, Wang GJ, Dai YQ, Zhang JJ, Zhang HF,Wang R, Mao CX , Li XY, Gong YL, Liu FY, Wang K. Preparation method for improving yield of fat dicarboxylic dihydrazide nucleating agent, CN Patent, CN 102976969 A
Hanna LA, Hendra PJ, Maddams W, Willis HA, Zichy V, Cudby MEA (1988) Vibrational spectroscopic study of structural changes in isotactic polypropylene below the melting point. Polymer 29(10):1843–1847
Okada K, Watanabe K, Urushihara T, Toda A, Hikosaka M (2007) Role of epitaxy of nucleating agent (NA) in nucleation mechanism of polymers. Polymer 48(1):401–408
Xing Q, Li RB, Dong X, Luo FL, Kuang X, Wang DJ, Zhang LY (2015) Enhanced crystallization rate of poly(L-lactide) mediated by a hydrazide compound: nucleating mechanism study. Macromol Chem Phys 216(10):1134–1145
Zhao SM, Chen FH, Huang YJ, Dong JY, Han CC (2014) Crystallization behaviors in the isotactic polypropylene/graphene composites. Polymer 55(16):4125–4135
Yang S, Li Y, Liang YY, Wang WJ, Luo Y, Xu JZ, Li ZM (2016) Graphene oxide induced isotactic polypropylene crystallization: role of structural reduction. RSC Adv 6(28):23930–22394
Acknowledgments
This work was financially supported by Hunan Provincial Natural Science Foundation of China (No. 2019JJ40294).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, YF., Mao, JJ. Effect of chemical structure of hydrazide compounds on nucleation effect in isotactic polypropylene. J Polym Res 26, 277 (2019). https://doi.org/10.1007/s10965-019-1970-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-019-1970-7