Abstract
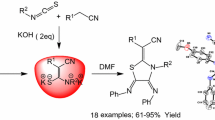
The reaction between N-substituted alkenylidene hydrazinecarbothioamides and two molar amounts of tetracyanoethylene (TCNE) in anhydrous THF at room temperature without using any catalyst affords (Z)-4-amino-3-((Z)substituted amino)-2-(substituted imino)-2,3-dihydrothiazole-5-carbonitriles and (Z)-(4-amino-5-cyano-thiazol-2(3H)-ylidene)carbonhydrazonoyl dicyanides. Rationales for these transformations are presented. The structures of the obtained products were confirmed via single-crystal X-ray analyses.
Graphic Abstract
Here, we synthesize (Z)-4-amino-3-amino-2-imino-2,3-dihydrothiazole-5-carbonitriles and (Z)-(4-amino-5-cyano-thiazol-2(3H)-ylidene)carbonhydrazonoyl dicyanides from the reaction of N-substituted alkenylidene hydrazinecarbothioamides with (TCNE) in anhydrous THF at room temperature without using any catalyst.









Similar content being viewed by others
References
J.-H. Park, M.I. El-Gamal, Y.S. Lee, C.-H. Oh, Eur. J. Med. Chem. 46, 5769 (2011)
C. Amit, A. Sheelmani, C. Singh, R.K.D. Payal, Adv. J. Pharm. Lif. Sci. Res. 2, 1 (2014)
R.D. Kamble, R.J. Meshram, S.V. Hese, R.A. More, S.S. Kamble, R.N. Gacche, B.S. Dawane, Comput. Bio. Chem. 61, 86 (2016)
B.S. Holla, K.V. Malini, B.S. Rao, B.K. Sarogini, N.S. Kumari, Eur. J. Med. Chem. 38, 313 (2003)
V. Jaishree, N. Ramdas, J. Sachin, B. Ramesh, J. Saudi Chem. Soc. 16, 371 (2012)
B. Sadek, M.M. Al-Tabakha, K.M.S. Fahelelbom, Molecules 16, 9386 (2011)
Y. Li, N. Bionda, R. Fleeman, H. Wang, A. Ozawa, R.A. Houghten, L. Shaw, Bioorg. Med. Chem. 24, 5633 (2016)
G. Samala, P.B. Devi, S. Saxena, N. Meda, P. Yogeeswari, D. Sriram, Bioorg. Med. Chem. 24, 1298 (2016)
C. Borelli, M. Schaller, M. Niewerth, K. Nocker, B. Baasner, D. Berg, R. Tiemann, K. Tietjen, B. Fugmann, S. Lang-Fugmann, H.C. Korting, Chemotherapy 54, 245 (2008)
K.M. Dawood, T.M.A. Eldebss, H.S. El-Zahabi, A.M.H. Yousef, Eur. J. Med. Chem. 102, 266 (2015)
C.B. Mishra, S. Kumari, M. Tiwari, Eur. J. Med. Chem. 92, 1 (2015)
Z.-Q. Sun, L.-X. Tu, F.-J. Zhuo, S.-X. Liu, Bioorg. Med. Chem. Lett. 26, 747 (2016)
D. Davyt, G. Serra, Mar. Drugs 8, 2755 (2010)
M.T. Chhabria, S. Patel, P. Modi, P.S. Brahmkshatriya, Curr. Top. Med. Chem. 16, 2841 (2016)
S. Nayak, S.L. Gaonkar, Mini Rev. Med. Chem. 19, 215 (2019)
A.Z. Halimehjani, L. Hasani, M.A. Alaei, M.R. Saidi, Tetrahedron Lett. 57, 883 (2016)
D. Zhao, S. Guo, X. Guo, G. Zhang, Y. Yu, Tetrahedron 72, 5285 (2016)
M. Xiabing, K. Ablajan, M. Obul, M. Seydimemet, R.R.L. Wenbo, Tetrahedron 72, 2349 (2016)
A.A. Hassan, A.A. Aly, S.M. Mostafa, D. Döpp, Arkivoc iii, 200 (2018)
A.A. Hassan, N.K. Mohamed, K.M.A. El-Shaieb, H.N. Tawfeek, S. Bräse, M. Nieger, Arkivoc vi, 163 (2016)
T. Freese, M. Nieger, J.C. Namyslo, A. Schmidt, Tetrahedron Lett. 60, 1272 (2019)
Z. Han, J. Lv, J. Zhang, Tetrahedron 75, 2162 (2019)
A.A. Hassan, F.F. Abdel-Latif, A.M.N. El-Din, S.M. Mostafa, M. Nieger, S. Bräse, Tetrahedron 68, 8487 (2012)
A.A. Aly, A.A. Hassan, M.A. Ameen, A.B. Brown, Tetrahedron Lett. 49, 4060 (2008)
A.A. Hassan, A.A. Aly, N.K. Mohamed, K.M. El Shaieb, M.M. Makhlouf, E.-S.M. Abdelhafez, S. Bräse, M. Nieger, K.N. Dalby, T.S. Kaoud, Bioorg. Chem. 85, 585 (2019)
A.A. Hassan, N.K. Mohamed, M.M. Makhlouf, S. Braese, M. Nieger, H. Hopf, Synthesis 48, 3134 (2016)
A.A. Hassan, F.F. Abdel-Latif, M.A. Aziz, S.M. Mostafa, S. Bräse, M. Nieger, Chem. Pap. 69, 973 (2015)
D.-C. Ilies, E. Pahontu, S. Shova, A. Gulea, T. Rosu, Polyhedron 51, 307 (2013)
A.A. Hassan, A.A. Aly, K.M. El-Shaieb, T.M. Bedair, S. Bräse, A.B. Brown, J. Heterocycl. Chem. 51, 674 (2014)
A.A. Hassan, K.M. El‐Shaieb, R.M. Shaker, D. Döpp, Heteroatom Chem. 16, 12 (2005)
G.M. Sheldrick, Acta Crystallographica A71 1, 3 (2015)
G.M. Sheldrick, Acta Crystallographica C71 1, 3 (2015)
Acknowledgements
Alaa A. Hassan is indebted to AvH foundation for the donation of a Shimadzu 408 IR instrument.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hassan, A.A., Aly, A.A., Mohamed, N.K. et al. Reactivity of N-substituted alkenylidene hydrazinecarbothioamides toward tetracyanoethylene, an efficient synthesis stereoselective 1,3-thiazole compounds. Res Chem Intermed 46, 1571–1585 (2020). https://doi.org/10.1007/s11164-019-04051-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-019-04051-4