Abstract
Electron paramagnetic resonance (EPR) spectroscopy is the ideal method of choice when detecting and studying the wide variety of paramagnetic oxygen-centred radicals. For simple diatomic radicals, such as the superoxide (O−2) or peroxy \( ({\text{ROO}}^{\bullet})\) species, the CW EPR profile (in particular the g-values) of these species can appear similar and indeed indistinguishable in some cases. Experiments using 17O-enriched oxygen, revealing a rich 17O hyperfine pattern, are therefore essential to distinguish between the two species. However, in many cases, particularly involving TiO2 photocatalysis, the peroxy-type \( ({\text{ROO}}^{\bullet})\) radicals or other intermediate species such as the [O−2…organic]-type adducts can be transient in nature and once again can produce similar g-values. In general terms, these reactive oxygen species (ROS) are formed and detected at low-temperature conditions. Hence, the application of EPR spectroscopy to studies of surface-stabilised oxygen-centred radicals must be performed under carefully selected conditions in order to confidently distinguish between the differing types of diatomic radicals, such as O−2, \( {\text{ROO}}^{\bullet}\) or [O−2…organic].
Similar content being viewed by others
Introduction
The photocatalytic activity of semiconductor metal oxides, such as titanium dioxide (TiO2), is of considerable interest because of their potential use in a wide range of applications such as water purification [1], solar energy conversion [2] and pollution control [3]. Upon absorption of a photon with energy equal to or greater than the band gap of the semiconductor, an electron/hole pair is generated in the bulk. These charge carriers migrate towards the catalyst surface where they can participate in redox reactions with adsorbed substrate molecules [4,5,6,7]. Whilst the bulk oxide properties, such as the optical band gap, are clearly related to catalytic activity, the surface properties are equally important as the reactions obviously occur at this interface. The successful application of metal oxides in photocatalytic reactions relies on very efficient electron-/hole-mediated transfer processes across the surface interface. An understanding of the interfacial chemistry at the molecular level, particularly the nature of surface intermediates which typically involve the formation of reactive oxygen species (ROS), is therefore considered central to the design of more improved photocatalysts.
The nature of the surface oxygen species formed on metal oxides is often dependent on surface morphology, and a vast majority of work in this field has been reported on well-defined single crystal surfaces, using steady-state surface science techniques [8]. However, surface intermediates can also be transient (i.e. thermally unstable) in character and can therefore be difficult to detect and identify using standard spectroscopic techniques. For paramagnetic oxygen species, electron paramagnetic resonance (EPR) spectroscopy is a vital tool in the characterisation of these oxygen-centred radicals and their subsequent reactivity over polycrystalline oxide surfaces. Several research groups have demonstrated how the superoxide radical anion can act as a probe of surface morphology, not only on TiO2 but also on other polycrystalline metal oxides [9,10,11,12,13,14,15,16,17,18]. Although the g-values are invaluable in providing important information about the radicals, particularly the surface speciation, the 17O hyperfine tensor is far more informative for determining the electronic structure. The use of oxygen isotopically enriched in 17O (I = 5/2) has been particularly valuable in identifying mononuclear [19] and molecular [20] surface oxygen species.
This detailed approach to characterising the radicals is essential when formulating a comprehensive description of the reactive oxygen species involved in surface reactions. For example, superoxide (O−2) and organoperoxy \( ({\text{ROO}}^{\bullet})\) radicals often produce similar g-values, easily resulting in a misinterpretation of the spectra and hence a mis-assignment of the radical. However, the 17O hyperfine pattern provides a clear and unambiguous means to distinguish between the two species [20]. On the other hand, many reactive oxygen species may be thermally unstable and therefore may not be readily detected in the EPR experiments. In such cases, low-temperature in situ methods are essential to fully inform the experimentalist in studying the surface reaction. In this manuscript, we will demonstrate these two key points by illustrating not only how EPR is used to discriminate between different O−2 species on oxide surfaces, but also how a class of paramagnetic ROS involved in oxidation reactions are only visible within a narrow low-temperature range (≈ 140–220 K) and therefore exemplify the need to perform the experiments and measurements under these low-temperature conditions.
Experimental
Materials
O−2on MoO3/SiO2: The samples of supported molybdenum were prepared by a grafting method [21], where MoCl5 was reacted with surface silanol groups in organic media according to the process:
After hydrolysis of the remaining chlorides, the catalyst was washed and dried in air at 120 °C. After transfer into quartz EPR tubes (4 mm thin wall, Wilmad-LabGlass), the samples were first preheated in oxygen at 600 °C then reduced at 600 °C under 100 Torr H2 for 30 min and finally evacuated to 1 × 10−5 Torr. Oxygen was adsorbed at 77 K at pressures of 10−2 to 10−1 using 16O2 or isotopically enriched 17O2 (63 atom % 17O, Icon Isotopes) to produce the O−2 superoxide ions.
O−2on Polypropylene: Powdered isotactic polypropylene was obtained from lCl Ltd. The samples were evacuated for 48 h at 100 °C and 1 × 10−5 Torr and then γ-irradiated in vacuo at room temperature with a 60Co source to a dose of 70 Mrad. The gaseous radiation products were evacuated and the samples transferred under vacuum in EPR tubes. Oxygen was added under 3 Torr to form the corresponding peroxy radicals. The labelled peroxy radicals were obtained by adsorption of 17O-enriched oxygen.
ROS on TiO2: The polycrystalline TiO2 (Degussa P25) was placed in an EPR cell and heated to 423 K under dynamic vacuum (10−4 Torr) overnight to remove any physisorbed water from the surface. The temperature was subsequently raised over 5 h to a final temperature of 773 K and left at this temperature for 1 h. This produced a non-stoichiometric, blue-coloured sample due to the excess of Ti3+ centres. The reduced sample was cooled prior to exposure to molecular oxygen and selected organic substrates. The organic substrates (including methanol, toluene and acetonitrile) were exposed to the sample at 77 K, followed by annealing to a series of elevated temperatures (200–260 K) for a period of 10 min. In some experiments, co-adsorbed mixtures of oxygen and the organic substrate were premixed in the vacuum manifold (with a ratio of 1:10) prior to exposure to the TiO2 surface at 298 K. All UV irradiations were performed at 77 K using a broadband (250–2500 nm) 1000-W UV lamp (Oriel Instruments, 66021), incorporating a water filter for removal of infrared frequencies.
EPR spectroscopy: The EPR spectra were obtained using a continuous wave (CW) Varian (E3 or CS – E109) or Bruker EMX spectrometer operating at ca. 9.3 GHz (X-band), with 100 kHz field modulation and optimised field modulation depth for maximum resolution of spectral features. All measurements were performed at low temperatures (T = 77 or 140 K) or room temperature (as reported in the figure captions).
Results and discussion
EPR characterisation of superoxide species on MoO3/SiO2
The superoxide radical can be easily formed by the exposure of a H2-reduced MoO3/SiO2 sample to molecular oxygen. After the reducing treatment in hydrogen, the grafted molybdenum sample exhibited a composite EPR spectrum due to Mo5+ ions, as detailed in the earlier work [22]. The adsorption of 16O2 at 77 K onto this reduced sample led to a large decrease in the Mo5+ signal and the corresponding appearance of a rhombic EPR signal characterised by the g-values of gzz = 2.017, gyy = 2.009 and gxx = 2.002. The reduction in the Mo5+ signal intensity and the appearance of the new EPR signal can be attributed to an electron transfer event from the reduced oxide surface to the adsorbed molecular oxygen, forming a surface-stabilised superoxide ion O−2 on Mo6+ [23, 24]. When similar experiments were repeated using 17O-enriched oxygen, the new EPR spectrum (Fig. 1) was produced which revealed a complex hyperfine structure that is far better resolved compared to previous reports for this system [23, 24]. The multiplet patterns arise from the hyperfine coupling of the superoxide ion with the I = 5/2 spin of the 17O nucleus. Remarkably, a similar EPR spectrum could also be obtained if a sample containing stabilised 16O−2 ions was subsequently exposed to 17O-enriched oxygen. This result indicates that an isotope exchange reaction takes place over the metal oxide surface, as described by Eqs. 1–2 as follows:
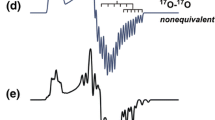
In previous reports of superoxide radicals stabilised on TiO2 or MgO surfaces [20], side-on bonding of the O−2 ions results in two equivalent oxygen nuclei (i.e. equal spin distribution over both atoms). The resulting EPR spectra in Fig. 1 contain two distinct sets of hyperfine multiplet patterns centred on the lowest gxx component [25, 26]. The first multiplet consists of six hyperfine lines with Axx = 213 MHz, indicating the presence of a singly labelled 16O17O− radical. The second multiplet pattern contains 11 hyperfine lines, assigned to a doubly labelled 17O17O− radical. As shown in Fig. 1, the present situation is more complex; indeed, careful analysis indicates that, despite some overlap in the hyperfine lines, two unique sets of six equally spaced lines appear clearly in the spectrum. Both of these sextet multiplet patterns are centred on the same gxx component and are independently characterised by hyperfine coupling values of Axx(i) = 238 and Axx(ii) = 201 MHz, respectively.
Two explanations may be possible for the observation of these two unique sextet multiplet patterns. The first case labelled (a) in Fig. 2 is that the hyperfine couplings arise from two distinct singly labelled O−2 anions stabilised on different surface sites, which experience different electrostatic potential interactions with the surface and this results in the distinct hyperfine couplings. In general, O−2 stabilised at different surface sites should lead to two unique g tensors. In particular, variations are typically observed in the gzz value, due to the sensitivity of this component to the non-degeneracy of the two πg antibonding orbitals originating from the oxide crystal field [20]. It is also likely that they will respond differently in their rate of formation, reactivity, saturation behaviour or thermal stability, as we have previously demonstrated [26]. It is important to note that this has not been observed in the present case. An alternative description labelled case (b) is based on two slightly inequivalent oxygen nuclei within the same O−2 radical, which could arise from an end-on binding mode to the oxide surface (Fig. 2).
The choice between the two previous explanations can be made after careful consideration of the outermost hyperfine lines arising from the doubly labelled 17O17O− species. Figure 3 gives a schematic illustration of the multiplet patterns that can be expected for the two cases (a) and (b), respectively. It is seen that in case (a), the total number of hyperfine lines amounts to [(2 × 6) + (2 × 11)] = 34, with a total spectral width of [10 × Axx(i)] assuming that Axx(i) > Axx(ii), where Axx(i) and Axx(ii) refer to the hyperfine splittings for labelled O−2 in the two types of sites. For case (b), the total number of hyperfine lines is [(2 × 6) + (6 x 6)] = 48 with a total spectral width of [5 × (Axx(i) + Axx(ii))], with Axx(i) > Axx(ii), assuming that Axx(i) and Axx(ii) refer to the inner and outer oxygen relative to the surface, respectively. In fact, the total number of hyperfine lines observed is expected to be smaller than the calculated maximum because of the overlap between the lines. Analysis of the outermost 17O17O− lines in Fig. 1 shows that the total spectral width is 2211 MHz (= 787 Gauss). Taking Axx(i) = 238 MHz and Axx(ii) = 201 MHz, as characterised from the two distinct sextet patterns, the total spectral width clearly closely corresponds with [5 × (238 + 201)] = 2195 MHz and not [10 × 201] = 2010 MHz. This result clearly indicates that for O−2 stabilised on MoO3/SiO2, there are two inequivalent oxygen nuclei in the same O−2 adsorbed at one type of surface site, corresponding to the situation described by case b).
The observation of oxygen isotopic exchange (OIE) over the MoO3/SiO2 surface is in agreement with previous reports on other oxides [27,28,29,30,31]. Indeed, the study of OIE is an effective way for understanding the reactivity of oxygen species on oxide surfaces but has previously been observed to involve exchange of surface oxygen atoms with atoms in the gas phase, via Eqs. 3–4 as follows:
EPR characterisation of superoxide species over γ-irradiated polypropylene
It is well known that γ-irradiation of polymers leads to chain scission events and ultimately an abundance of reactive carbon-based radicals. These radicals can readily react with molecular oxygen (at least for the surface accessible radicals resulting from rapid O2 diffusion through the polymer). Upon γ-irradiation of isotactic polypropylene, followed by exposure to molecular oxygen, the EPR spectrum shown in Fig. 4 is observed at 77 K. The predominant axial spectrum is characterised by the g-values of gzz = 2.0351, gyy = 2.0081 and gxx = 2.0024, and this signal can be readily assigned to stabilised peroxy radicals \( ({\text{ROO}}^{\bullet})\), in agreement with previous reports [32,33,34,35].
When the same experiment is performed in the presence of 17O-enriched oxygen, a more complex EPR spectrum is obtained with a rich hyperfine multiplet pattern (Fig. 5). Detailed analysis of the hyperfine coupling centred on the gxx component resolves two unique multiplets, characterised by Axx(I) = 276 MHz (98.5 Gauss) and Axx(II) = 168 MHz (60 Gauss). The substantial difference between the magnitudes of the two hyperfine couplings is indicative of two inequivalent oxygen atoms, indicating an end-on interaction of the molecular oxygen with the carbon-based \( {\text{R}}^{\bullet}\) radicals generated in the polymer during γ-irradiation. The hyperfine couplings, Axx(I) and Axx(II), are therefore assigned to the inner and outer oxygens atoms, respectively, within this diatomic \( {\text{ROO}}^{\bullet}\) centre. Notably, when the sample containing surface-stabilised peroxy radicals is annealed between 77 and 298 K, no change in the EPR spectrum is detected. This observation indicates the thermal stability of the peroxy radicals in the polymer material, where extensive mobility of the radical is not permitted unlike the situation observed on metal oxide surfaces where the radicals are only weakly adsorbed.
EPR characterisation of superoxide reactivity and ROS on TiO2
In the previous section, describing the EPR characterisation of the O−2 centres, the radical in question on the MoO3/SiO2 surface was thermally stable. This is a frequently observed case for O−2 on several other oxide surfaces including TiO2, regardless of the original method used to generate the radical [9, 20, 36, 37]. However, surface intermediates, in particular reactive oxygen species (ROS), can also be transient (i.e. thermally unstable) in nature and can therefore be difficult to directly detect and study. In many cases, it is well known that oxygen-based radicals (including O−2) on TiO2 are essential to many photooxidation processes [38,39,40]. Indeed, EPR has often been used to monitor the reactivity of the oxygen-centred radicals in various oxidation-based surface reactions [20]. In previous work, we have demonstrated how a series of transient organoperoxy radicals \( ({\text{ROO}}^{\bullet})\) can play a contributory role in the decomposition of acetone and acetonitrile over TiO2 [41, 42], but these species are only stable and therefore visible at temperatures lower than 200 K (unlike O−2 which is readily detected at room temperature). Therefore, in this section, we will evidence how organic substrates react with superoxide radicals over polycrystalline TiO2 surfaces under dark conditions leading to transient ROS.
Similar to the case reported for acetone [41], a thermally unstable ROS was observed when acetonitrile (CH3CN) was exposed to a TiO2 sample that contained preformed O−2 radicals (i.e. O−2/TiO2); the O−2 centres were readily formed by exposure of molecular oxygen to thermally reduced TiO2 [42]. The ROS was identified as a transient [O−2…CH3CN] adduct, characterised by the g-values listed in Table 1. An analogous surface species was also observed with acetone, i.e. [O−2…CH3COCH3] [41]. These species are thermally unstable and decompose at temperatures T > 220 K. During the reaction between surface O−2 and acetonitrile, a second radical is also formed and identified as the hydroperoxy \( ({{\text{H}}}{\text{O}}_2^{\bullet})\) species, with spin Hamiltonian parameters of g1 = 2.028, g2 = 2.010, g3 = 2.004, HA1 = 34.1 MHz (1.2 mT), HA2 = 28.1 MHz (1.0 mT) and HA3 = 28.0 MHz (1.0 mT) [42]. We therefore wanted to explore whether these transient surface adducts, abbreviated hereafter using the generic label [O−2…organic], are common to other organic substrates, such as methanol and toluene.
To probe whether a transient [O−2…CH3OH] surface adduct could be formed, analogous to the [O−2…CH3CN] [42] and [O−2…CH3COCH3] [41] adducts, methanol (10 Torr) was exposed to a O−2/TiO2 sample. Room temperature exposure of the methanol vapours to this sample resulted in the immediate disappearance of the O−2 EPR signals. However, when the methanol was admitted to O−2/TiO2 at 77 K, followed by thermal annealing between T = 200 K and 240 K, a gradual decrease in the O−2 signal intensity was observed. Simultaneously, a new peak emerged in the gzz region with g ~ 2.028 (Fig. 6), due to the formation of a new radical centre.
As described previously by us [26, 43], the site-specific O−2 centres on TiO2 (labelled sites I, II and III in Fig. 6) slowly decay due to reaction of O−2 with the organic substrate (methanol in this case), accompanied by the appearance of the new signal at g ~ 2.028. This latter signal grows in intensity as the temperature is increased, before decaying at T > 220 K. The reactivity between CH3OH and O−2 appears to be non-site selective, analogous to the situation observed with acetonitrile where all three O−2 sites I, II and III react readily with the substrate. The peaks labelled * in Fig. 6 are assigned to the \( {{\text{H}}}{\text{O}}_2^{\bullet}\) species, possessing similar g- and HA-values to those given above for acetonitrile. This assignment to \( {{\text{H}}}{\text{O}}_2^{\bullet}\) was confirmed in the current case using fully deuterated methanol (CD3OD); in this latter case, the superhyperfine peaks arising from the \( ^2{{\text{H}}}{\text{O}}_2^{\bullet}\) were not visible in the EPR spectrum, as expected due to the smaller magnetic moment of 2H compared to 1H.
The above experiment with methanol was also repeated using toluene, and the resulting EPR spectra are shown in Fig. 7. Addition of toluene to O−2/TiO2 at room temperature resulted in the complete loss of the superoxide signals, as the toluene reacts with and destroys the O−2 centres. However, upon addition of toluene at 77 K, followed by gradual annealing to elevated temperatures (200–265 K), a gradual decrease in the O−2 signal intensity accompanied by the gradual appearance of a new peak in the gzz region at g ~ 2.025 was observed (Fig. 7). Unlike the previous methanol case, the reactivity of toluene with O−2 now appears to be site specific. As evident from Fig. 7, the O−2 site I species (previously assigned to a superoxide centre stabilised in close proximity to a surface vacancy site, labelled [Vac…O−2] [41]) reacts preferentially with toluene relative to O−2 sites II–III (the non-vacancy stabilised O−2 centres) [41]. The reason why [Vac…O−2] would react differently compared to the non-vacancy stabilised O−2 centres with toluene compared to methanol is not currently understood, but clearly these results show the interesting variable behaviour of heterogeneous site-specific O−2 reactivity on TiO2. The \( {{\text{H}}}{\text{O}}_2^{\bullet}\) species is also formed when toluene reacts with O−2/TiO2, and once again this assignment was confirmed using fully deuterated toluene resulting in the loss of HA signals from the EPR spectrum (Fig. 7). The signal at g ~ 2.025 appears most intense at T = 265 K and can be assigned to the transient [O−2…C6H5CH3] adduct. This species then decays at more elevated temperatures above 265 K.
Owing to the considerable overlap of the g components for both O−2 and [O−2…C6H5CH3], the precise assignment of the g1 and g2 components for the latter adduct is difficult to extract. As a result, the experiment was repeated using TiO2 thermally reduced at lower temperatures (623 K). The lower reduction temperature decreases the extent of O−2 speciation on TiO2, so that only one O−2 centre (site I with g1 = 2.019, g2 = 2.011 and g3 = 2.005) predominates, thereby considerably simplifying the spectrum. Toluene was then exposed to the site I dominated O−2/TiO2 sample, and the resulting spectra are shown in Fig. 8. Owing to the improved resolution, the experimental spectrum for [O−2…C6H5CH3] could be simulated with greater confidence; the extracted spin Hamiltonian parameters are listed in Table 1.
CW X-band EPR spectra (130 K) of (a) O−2 formed on a thermally reduced TiO2 sample (reduced at 623 K) and after exposure of this sample to toluene (10 Torr) at 77 K followed by annealing to (b) 130, (c) 220, (d) 230, (e) 240, (f) 250 and (g) 260 K. The g-values for O−2 and [O−2…C6H5CH3] are highlighted with the dash-dot and dash lines, respectively
In the above cases for methanol and toluene (and previously reported for acetonitrile [42] and acetone [41]), the organic substrates were exposed to the thermally reduced TiO2 sample containing the O−2 centres. However, we were also interested in exploring whether the route to O−2 formation has any bearing on the subsequent reactivity of O−2. In the next series of experiments, the O−2 centres were therefore generated over TiO2 using UV irradiation, i.e. to test whether O−2(thermal)/TiO2 behaves and reacts differently with the organic substrates compared to O−2(UV)/TiO2. The results obtained by reacting the O−2 with acetonitrile are shown in Fig. 9.
CW X-band EPR spectra (130 K) of (a) O−2 formed photochemically by UV irradiation of TiO2 containing adsorbed molecular O2. After O−2(UV) formation, the excess O2 was evacuated prior to exposure of this sample to acetonitrile (7.3 Torr) at 77 K followed by annealing to (b) 140, (c) 190, (d) 200, (e) 210, (f) 215, (g) 220 and (h) 230 K
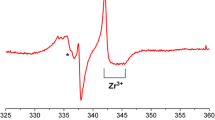
The EPR spectrum of the UV-generated O−2 species on TiO2 is shown in Fig. 9a. This signal is considerably more complex compared to the O−2(thermal)/TiO2 (see Figs. 6a, 7a, 8a, for example). The photochemically generated superoxide O−2 centre has the spin Hamiltonian parameters of gxx ~ 2.004, gyy = 2.011 and at least six observable gzz values of 2.017 (I’), 2.019 (I), 2.020 (II’), 2.023 (II), 2.025 (II’’) and 2.026 (III). The extra sites I’ and II’ (with gzz = 2.017 and 2.025) are due to additional stabilisation sites on the TiO2 surface which are not available on the thermally reduced surface. Additional peaks in the spectrum (marked #) are due to traces of interstitial photo-generated Ti3+ centres (Fig. 9). Acetonitrile was subsequently added to this O−2(UV)/TiO2 sample at 77 K. Upon annealing under the acetonitrile atmosphere, the O−2 signal intensity decreased, and this was accompanied by the appearance of a new signal at g ~ 2.032 (Fig. 9b–h). This new signal was first observed at T ~ 210 K and begins to decay irreversibly at T > 250 K; this signal can once again be assigned to the transient [O−2…CH3CN] adduct.
The main difference between the thermally treated [42] and photoirradiated samples (Fig. 9) is the lower overall intensity of the [O−2…CH3CN] adduct on the photoirradiated sample. The reason for this is simply due to the lower initial abundance of UV-generated O−2. Nevertheless, there appears to be no appreciable difference observed in the reactivity of acetonitrile with O−2(thermal)/TiO2 and O−2(UV)/TiO2. The formation of the radical species [O−2…CH3CN] and \( {{\text{H}}}{\text{O}}_2^{\bullet}\) is observed in both cases. This is an important result as it shows that these transient [O−2…CH3CN] adducts can be formed from O−2 radicals generated under both thermal and photochemical conditions.
As previously evidenced by us using 17O-labelled oxygen [26], the O−2 centres in the adducts have equivalent oxygen atoms, unlike the inequivalent spin densities that would be expected in the peroxy case [42]. This indicates a side-on manner of interaction between O−2 and the organic substrates. The resulting series of EPR spectra for these transient [O−2…organic] adducts with acetone, acetonitrile, methanol and toluene are summarised in Fig. 10. We have previously postulated that the formation of the transient adducts arises from the nucleophilic attack by the O−2 anion on the electrophilic carbon atom of the organic substrate [41]. If the strength of this interaction is dependent on the electrophilic nature of the carbon to which it is attached, the series of substrates presented in Fig. 10 can provide insights into how this interaction is affected. The relative electron-withdrawing power of the functional groups to which the CH3 group is attached is C6H5 (toluene) < OH (methanol) < CO (acetone) ~ CN (acetonitrile). The g1 values for the resulting [O−2…organic] species then mirror this trend, as indicated in Fig. 10 and Table 1.
In all of the above-cited experiments, the organic substrates (methanol and toluene) were exposed to TiO2-containing surface-stabilised O−2 centres. However, previous work from our group revealed how direct UV irradiation of TiO2 containing co-adsorbed mixtures of O2/organic substrates can yield a series of transient organoperoxy and peroxyacyl reactive oxygen species in the presence of ketones [41, 43] and aldehydes [44] which are formed via hole-mediated processes (vide infra). To explore this photochemistry in greater detail, a co-mixture of acetonitrile plus O2 was added to a TiO2 sample in a ratio of 10:1 (total pressure = 15 Torr) to ensure the organic substrate was in excess. The sample was then cooled to 77 K and exposed to UV radiation at this temperature for 30 min. After annealing the sample to 130 K, the excess gas was removed before recording the EPR spectrum (Fig. 11). Similar experiments were also performed using methanol and toluene with similar results (data not shown here). Confirmation of the assignment to the peroxy radicals with methanol and toluene is currently underway in our laboratory using enriched 17O2.
The EPR spectrum of UV-radiated TiO2 containing acetonitrile–O2 is dominated by an orthorhombic signal with g-values of g1 = 2.034, g2 = 2.010 and g3 = 2.004; additional signals at g = 1.989 and g ~ 1.972 (labelled * in Fig. 11) are also formed, which can be easily assigned to bulk Ti3+ cations at substitutional and lattice sites (generated during low-temperature irradiation of the TiO2) [45,46,47]. The orthorhombic signal is thermally unstable; upon warming to elevated temperatures (T > 180 K), the signal intensity progressively decreases (not shown here) until eventually it is completely lost at 298 K. However, this signal can be regenerated by admission of an additional dose of CH3CN/O2 followed by re-irradiation of the sample at 77 K. The signal was only observed with a high CH3CN/O2 ratio; if molecular oxygen is abundant, a signal corresponding to the photo-generated O−2 centres is observed.
The spin Hamiltonian parameters for this new signal are somewhat reminiscent of the earlier [O−2…CH3CN] adduct (Table 1), with one notable exception: the g1 value of 2.034. The overall spectral profile is in fact identical to that of the organoperoxy species formed with acetone and other ketones [41, 42]. These organoperoxy-type intermediates, formed after irradiation of a co-adsorbed mixture of organic–O2 have been identified over TiO2 and are typically characterised by the spin Hamiltonian parameters of g1 = 2.034, g2 = 2.010 and g3 = 2.003. These values compare favourably with those observed here (Fig. 11); therefore, taking into consideration the similar thermal behaviour, this new species can be readily assigned to an organoperoxy-type radical \( {\text{NCCH}}_2{\text{OO}}^{\bullet}\). Irradiation of TiO2 results in the generation of an electron (e−)–hole (h+) pair. These can either recombine in the bulk or migrate to the surface of TiO2, where they participate in surface reactions with adsorbed species. The electron can migrate to the surface to form a Ti3+ centre, whereas the holes are trapped at surface O2− sites to give O−. The acetonitrile is adsorbed on the TiO2 surface at Lewis acid sites and is quickly deprotonated by H+ transfer to produce a hydroxyl group and \({\text{NCCH}}_{2}^{ \bullet }\):
The resulting radical cation can then react with molecular oxygen forming the peroxy radical:
A similar mechanism for the formation of these organoperoxy \( ({\text{ROO}}^{\bullet})\)-type radicals has been identified in the literature for the photooxidation of ketones [41] and ethylene [48] on TiO2; in the latter case, the g-values were g1 = 2.034, g2 = 2.010 and g3 = 2.001. In the present work, similar EPR spectra were also obtained using methanol and toluene (Table 1).
It is therefore clear that a series of transient organoperoxy radicals \( ({\text{ROO}}^{\bullet})\) can be formed after UV irradiation of an organic–O2 mixture (organic substrate = acetonitrile, acetone, methanol and toluene) over TiO2. These radicals are formed by hole-mediated processes, generating organic radical anions which rapidly react with molecular oxygen to form the resulting organoperoxy centres. The transient species all decay above T > 190 K, but can be easily regenerated and all produce analogous g-values. Unlike the aforementioned [O−2…organic] centres with characteristic and independent g1 values (Table 1), the g1 value of 2.034 is constant and independent of the organic substrate used. Sevilla et al. [49] showed that different organic substituents (R) have little effect on the reactivity and structure of \( {\text{ROO}}^{\bullet}\) peroxy radicals with the g-values for carbon-based peroxy radicals varying only slightly for different substituents. The nature of the R substituent, however, can affect the stability of the radical, and this may explain the different signal intensities which we observed for these radicals using the different organic substrates, i.e. the signals were weaker for methanol and toluene.
The thermal instability of these \( {\text{ROO}}^{\bullet}\) intermediates highlights the necessity to experimentally undertake in situ (EPR spectroscopy) measurements as other spectroscopic techniques may be unable to provide this low-temperature identification. The identification of these peroxy intermediates is also important as it shows how different surface treatments can result in the formation of different species. The organoperoxy radicals have been identified with aldehydes [44], ketones [41, 43], acetonitrile [42] and in this work with alcohols and toluene, indicating that this type of intermediate plays a key role in photooxidation processes using a variety of substrates.
Conclusions
The superoxide radical anion (O−2) is readily formed over MoO3/SiO2 by the exposure of the reduced sample to molecular oxygen, as widely reported. However, whilst the CW EPR spectrum of the unlabelled species is consistent with a surface 16O−2 centre, analysis of the spectrum obtained using 17O-enriched oxygen is necessary to fully interpret the nature of the radical which reveals the presence of an end-on bonded O−2 species. The same 17O−2 species could be formed from 16O−2 by the exposure of the sample to 17O-enriched molecular oxygen in an isotope exchange reaction. In principle, whilst side-on and end-on bonded O−2 species can exist on MoO3/SiO2, the latter species appear to dominate.
Paramagnetic oxygen-based radicals are also readily formed when molecular oxygen is exposed to γ-irradiated polypropylene. In this case, the CW EPR signal has notably broad linewidths. The profile of the spectrum is analogous to that expected of a generic ROS entity, with g1 = 2.035, g2 = 2.008 and g3 = 2.002, but once again only a definitive assignment to an end-on peroxy radical \( ({\text{ROO}}^{\bullet})\) can be made using 17O hyperfine pattern.
A number of oxygen-centred radicals have been proposed to play a key role in the degradation of organic substrates over TiO2 under dark conditions or under UV irradiation. Here, we have shown how the decomposition of acetonitrile, methanol and toluene can occur over O−2/TiO2. The reaction of the organic substrates with O−2 results in the production of a transient and thermally unstable (above 240 K) surface adduct, labelled [O−2…organic]. These adducts are characterised by the EPR g-values of g1 = 2.031-2.025, g2 = 2.010 and g3 = 2.003, and similar to previously published data appear to possess a side-on arrangement of the organic molecule with respect to the two equivalent oxygen atoms of O−2. Additionally, a hydroperoxy \( ({{\text{H}}}{\text{O}}_2^{\bullet})\) radical was also formed under these experimental conditions. This [O−2…organic] adduct can be formed regardless of how the O−2 radical was originally generated.
Furthermore, a series of thermally unstable surface-organoperoxy-type radical intermediates were also identified following UV irradiation of a series of co-adsorbed organic–O2 mixtures. These transient radicals \( ({\text{NCCH}}_2{\text{OO}}^{\bullet},{\text{HOCH}}_2{\text{OO}}^{\bullet}, {\text{C}}_7{\text{H}}_7{\text{OO}}^{\bullet})\) produced after UV irradiation all possessed analogous g-values (g1 = 2.034, g2 = 2.007, g3 = 2.001). The signal intensities for the \( {\text{HOCH}}_2{\text{OO}}^{\bullet}\) and \( {\text{C}}_7{\text{H}}_7{\text{OO}}^{\bullet}\) radicals were much weaker compared to those of the \( {\text{NCCH}}_2{\text{OO}}^{\bullet}\) species (possibly due to the different stabilities of the respective radicals and the different competing reactions that can occur with these substrates over the TiO2 surface).
The work presented in this paper demonstrates the complexity of organic substrate decomposition with ROS over TiO2. It is clear that these substrates can react and degrade under dark conditions (non-radiative) via a reaction with surface O−2 radicals forming transient [O−2…organic] adducts, or via UV irradiation conditions where hole-mediated processes lead to the formation of \( {\text{ROO}}^{\bullet}\) species. In all cases, the nature of the transient reactive oxygen species (ROS) in TiO2 photocatalysis is very important and highlights the need to perform low-temperature in situ measurements to obtain a complete picture on the oxidation radical steps.
References
Y. Yang, J. Shin, J.T. Jasper, M.R. Hoffmann, Environ. Sci. Tech. 50, 8780 (2016)
B. O’Regan, M. Graetzel, Nature 353, 737 (1991)
J. Schneider, M. Matsuoka, M. Takeuchi, J. Zhang, Y. Horiuchi, M. Anpo, D.W. Bahnemann, Chem. Rev. 114, 19 (2014)
T.L. Thompson, J.T. Yates, Chem. Rev. 106, 4428 (2006)
A. Kudo, Y. Miseki, Chem. Soc. Rev. 38, 253 (2009)
K. Honda, A. Fujishima, Nature 238, 37 (1972)
R.F. Howe, M. Gratzel, J. Phys. Chem. 91, 3906 (1987)
U. Diebold, Surf. Sci. Rep. 48, 53 (2003)
M. Chiesa, E. Giamello, M. Che, Chem. Rev. 110, 1320 (2010)
J.M. Coronado, J. Soria, Catal. Today 123, 37 (2007)
C. Murata, T. Hattori, H. Yoshida, J. Catal. 231, 292 (2005)
E. Giamello, E. Garrone, P. Ugliengo, J. Chem. Soc. Faraday Trans. 85, 1373 (1989)
E. Giamello, E. Garrone, P. Ugliengo, A.J. Che, J. Tench, J. Chem. Soc. Faraday Trans. 85, 3987 (1989)
M. Chiesa, M.C. Paganini, E. Giamello, C. Di Valentin, G. Pacchioni, Acc. Chem. Res. 39, 861 (2006)
M. Chiesa, M.C. Paganini, G. Spoto, E. Giamello, C. Di Valentin, A. Del Vitto, G. Pacchioni, J. Phys. Chem. B 109, 7314 (2005)
M. Chiesa, M.C. Paganini, E. Giamello, D.M. Murphy, J. Phys. Chem. B 105, 10457 (2001)
M. Anpo, M. Che, B. Fubini, E. Garrone, E. Giamello, M.C. Paganini, Top. Catal. 8, 189 (1999)
K. Sobanska, A. Krasowska, T. Mazur, K. Podolska-Serafin, P. Pietrzyk, Z. Sojka, Top. Catal. 58, 796 (2015)
M. Che, A.L. Tench, Adv. Catal. 31, 77 (1982)
M. Che, A.L. Tench, Adv. Catal. 32, 1 (1983)
C. Louis, M. Che, F. Boson-Verduras, J. Chem. Phys. 79, 000 (1982)
M. Dufaux, M. Che, C. Naccache, Compt Rend. de Seanc. de l’Academie des Sci. C 268, 2255 (1969)
Y.B. Taarit, J.H. Lunsford, J. Phys. Chem. 77, 780 (1973)
M. Shiotani, G. Moro, J.H. Freed, J. Chem. Phys 74, 2616 (1981)
A.J. Tench, P. Holroyd, Chem. Commun. 8, 471 (1968)
E. Carter, A.F. Carley, D.M. Murphy, J. Phys. Chem. C 111, 10630 (2007)
R.V. Mikhaylov, A.A. Lisachenko, V.V. Titov, J. Phys. Chem. C 116, 23332 (2012)
R.V. Mikhaylov, N.I. Glazkova, K.V. Nikitin, J. Photochem. Photobiol. A 332, 554 (2017)
H. Courbon, M. Formenti, P. Pichat, J. Phys. Chem. 81, 550 (1977)
X. Pang, C. Chen, H. Ji, Y. Che, W. Ma, J. Zhao, Molecules 19, 16291 (2014)
S. Sato, J. Phys. Chem. 91, 2895 (1987)
D. Olivier, C. Marachi, M. Che, J. Chem. Phys. 72, 3348 (1980)
D. Olivier, C. Marachi, M. Che, J. Chem. Phys. 71, 4688 (1979)
M. Che, A.J. Tench, J. Chem. Phys. 64, 2370 (1976)
M. Che, A.J. Tench, J. Polym. Sci. C Polym. Lett. 13, 345 (1975)
M. Chiesa, E. Giamello, M.C. Paganini, Z. Sojka, D.M. Murphy, J. Chem. Phys. 116, 4266 (2002)
A.L. Attwood, D.M. Murphy, J.L. Edwards, T.A. Egerton, R.W. Harrison, Res. Chem. Intermed. 29, 449 (2003)
S.B. Kim, H.T. Hwang, S.C. Hong, Chemosphere 48, 437 (2002)
R. Nakamura, S. Sato, J. Phys. Chem. B 106, 5893 (2002)
M.A. Henderson, C.L. Perkins, C.H.F. Peden, U. Diebold, J. Phys. Chem. B 103, 5328 (1999)
E. Carter, A.F. Carley, D.M. Murphy, ChemPhysChem 8, 113 (2007)
J. Green, E. Carter, D.M. Murphy, Res. Chem. Intermed. 35, 145 (2009)
A. Attwood, J. Edwards, C.C. Rowlands, D.M. Murphy, J. Phys. Chem. A 107, 1779 (2003)
C.A. Jenkins, D.M. Murphy, J. Phys. Chem. B 103, 1019 (1999)
D.C. Hurum, K.A. Gray, T. Rajh, M.C. Thurnauer, J. Phys. Chem. B 109, 977 (2005)
S. Livraghi, M. Chiesa, M. Cristina, E. Giamello, J. Phys. Chem. C 115, 25413 (2011)
S. Livraghi, M. Rolando, S. Maurelli, M. Chiesa, M. Cristina, E. Giamello, J. Phys. Chem. C 118, 22141 (2014)
A.R. Gonzalez-Elipe, M. Che, J. De Chim. Phys. Phys. Chim. Biol. 79, 355 (1982)
M.D. Sevilla, D. Becker, M.Y. Yan, J. Chem. Soc. Faraday Trans. 86, 3279 (1990)
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This manuscript is dedicated to, and in memory of, the late Prof Michel Che.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Richards, E., Murphy, D.M. & Che, M. An EPR characterisation of stable and transient reactive oxygen species formed under radiative and non-radiative conditions. Res Chem Intermed 45, 5763–5779 (2019). https://doi.org/10.1007/s11164-019-04001-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-019-04001-0