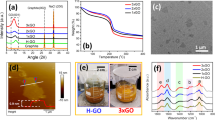
Abstract
A sustainable continuous-flow protocol for the conversion of aryl amine to unsymmetrical thioether is described. This technique is a two-step process involving graphene oxide (GO) catalyzed diazotization followed by the reaction with aryl/alkyl thiols. The continuous-flow conditions afford the desired thioethers in very good yields, effectively suppressing formation of possible disulfide, a common by-product in conventional process. The flow reaction is carried out under ambient conditions, applied to a variety of aryl amines and aryl/alkyl thiols and found to be scalable. The catalytic activity of the GO bed under continuous-flow conditions is found within standard time range and recyclable for ten consecutive runs without any loss of its performance.

Continuous-flow technique is applied in graphene oxide (GO) catalyzed conversion of aryl amine to unsymmetrical thioethers.





Similar content being viewed by others
References
Plutschack MB, Pieber B, Gilmore K, Seeberger PH (2017) The hitchhiker’s guide to flow chemistry. Chem Rev 117:11796–11893
Akwi FM, Watts P (2018) Continuous flow chemistry: where are we now? Recent applications, challenges and limitations. Chem Commun 54:13894–13928
Noël T, Su Y, Hessel V (2015) In: Noël T (ed) Organometallic flow chemistry. Germany, Springer
Oger N, Le Grognec E, Felpin F-X (2015) Handling diazonium salts in flow for organic and material chemistry. Org Chem Front 2:590–614
Sahoo HR, Kralj JG, Jensen KF (2007) Multistep continuous-flow microchemical synthesis involving multiple reactions and separations. Angew Chem Int Ed 46:5704–5708
Yoshida J-I, Nagaki A, Yamada D (2013) Continuous-flow synthesis. Drug Discov Today Technol 10:e53–e59
Kockmann N, Thenée P, Fleischer-Trebes C, Laudadio G, Noël T (2017) Safety assessment in development and operation of modular continuous-flow processes. React Chem Eng 2:258–280
Dreyer DR, Jia HP, Bielawski CW (2010) Graphene oxide: a convenient carbocatalyst for facilitating oxidation and hydration reactions. Angew Chem Int Ed 49:6813–6816
Dreyer DR, Park S, Bielawski CW, Ruoff RS (2010) The chemistry of graphene oxide. Chem Soc Rev 39:228–240
Mohammadi O, Golestanzadeh M, Abdouss M (2017) Recent advances in organic reactions catalyzed by graphene oxide and sulfonated graphene as heterogeneous nanocatalysts: a review. New J Chem 41:11471–11497
Navalon S, Dhakshinamoorthy A, Alvaro M, Garcia H (2014) Carbocatalysis by graphene-based materials. Chem Rev 114:6179–6212
Dreyer DR, Todd AD, Bielawski CW (2014) Harnessing the chemistry of graphene oxide. Chem Soc Rev 43:5288–5301
Bhattacharya S, Ghosh P, Basu B (2018) Graphene oxide (GO) catalyzed transamidation of aliphatic amides: an efficient metal-free procedure. Tetrahedron Lett 59:899–903
Choudhury P, Roy B, Basu B (2017) Sustainable and site-selective C−H Sulfenylation of aromatic compounds with thiol using catalytic graphene oxide and NaI. Asian J Org Chem 6:1569–1574
Jia HP, Dreyer DR, Bielawski CW (2011) Graphite oxide as an auto-tandem oxidation–hydration–aldol coupling catalyst. Adv Synth Catal 353:528–532
Gawande MB, Goswami A, Felpin F-X, Asefa T, Huang X, Silva R, Zou X, Zboril R, Varma RS (2016) Cu and cu-based nanoparticles: synthesis and applications in catalysis. Chem Rev 116:3722–3811
Lee CF, Liu YC, Badsara SS (2014) Transition-metal-catalyzed C–S bond coupling reaction. Chem Asian J 9:706–722
He L, Qiu G, Gao Y, Wu J (2014) Removal of amino groups from anilines through diazonium salt-based reactions. Org Biomol Chem 12:6965–6971
Mo F, Dong G, Zhang Y, Wang J (2013) Recent applications of arene diazonium salts in organic synthesis. Org Biomol Chem 11:1582–1593
Roglans A, Pla-Quintana A, Moreno-Manas M (2006) Diazonium salts as substrates in palladium-catalyzed cross-coupling reactions. Chem Rev 106:4622–4643
Stadler O (1884) Zur Kenntniss der Merkaptane. Ber Dtsch Chem Ges 17:2075–2081
Ziegler J (1890) Ueber eine Methode zur Darstellung aromatischer Sulfide von bestimmter Constitution und das Thioxanthon. Ber Dtsch Chem Ges 23:2469–2472
Wang X, Cuny GD, Noël T (2013) A mild, one-pot Stadler–Ziegler synthesis of arylsulfides facilitated by photoredox catalysis in batch and continuous-flow. Angew Chem Int Ed 52:7860–7864
Shieh Y-C, Du K, Basha RS, Xue Y-J, Shih B-H, Li L, Lee C-F (2019) Syntheses of Thioethers and selenide ethers from anilines. J Org Chem 84:6223–6231
Hong B, Lee J, Lee A (2017) Visible-light-promoted synthesis of diaryl sulfides under air. Tetrahedron Lett 58:2809–2812
Li Y, Pu J, Jiang X (2014) A highly efficient cu-catalyzed S-transfer reaction: from amine to sulfide. Org Lett 16:2692–2695
Bottecchia C, Rubens M, Gunnoo SB, Hessel V, Madder A, Noël T (2017) Visible-light-mediated selective Arylation of cysteine in batch and flow. Angew Chem Int Ed 56:12702–12707
Koziakov D, Majek M, von Wangelin AJ (2016) Metal-free radical thiolations mediated by very weak bases. Org Biomol Chem 14:11347–11352
Mukherjee N, Chatterjee T, Ranu BC (2013) Reaction under ball-milling: solvent-, ligand-, and metal-free synthesis of unsymmetrical diaryl chalcogenides. J Org Chem 78:11110–11114
Kundu D, Ahammed S, Ranu BC (2012) Microwave-assisted reaction of aryl diazonium fluoroborate and diaryl dichalcogenides in dimethyl carbonate: a general procedure for the synthesis of unsymmetrical diaryl chalcogenides. Green Chem 14:2024–2030
Barbero M, Degani I, Diulgheroff N, Dughera S, Fochi R, Migliaccio M (2000) Alkyl-and arylthiodediazoniations of dry arenediazonium o-benzenedisulfonimides. Efficient and safe modifications of the Stadler and Ziegler reactions to prepare alkyl aryl and diaryl sulfides. J Org Chem 65:5600–5608
Roy B, Ghosh S, Ghosh P, Basu B (2015) Graphene oxide (GO) or reduced graphene oxide (rGO): efficient catalysts for one-pot metal-free synthesis of quinoxalines from 2-nitroaniline. Tetrahedron Lett 56:6762–6767
Bochare MD, Degani MS (2017) Polyethylene glycol nitrite (PEG-ONO) as a novel diazotizing agent. ACS Sustain Chem Eng 5:3716–3720
M-j B, G-p L, Cai C (2015) Ascorbic acid promoted metal-free synthesis of aryl sulfides with anilines Nitrosated in situ by tert-butyl nitrite. Synlett 26:1841–1846
Hari DP, Schroll P, König B (2012) Metal-free, visible-light-mediated direct C–H arylation of heteroarenes with aryl diazonium salts. J Am Chem Soc 134:2958–2961
Voylov D, Saito T, Lokitz B, Uhrig D, Wang Y, Agapov A, Holt A, Bocharova V, Kisliuk A, Sokolov AP (2016) Graphene oxide as a radical initiator: free radical and controlled radical polymerization of sodium 4-vinylbenzenesulfonate with graphene oxide. ACS Macro Lett 5:199–202
Qiu Y, Wang Z, Owens AC, Kulaots I, Chen Y, Kane AB, Hurt RH (2014) Antioxidant chemistry of graphene-based materials and its role in oxidation protection technology. Nanoscale 6:11744–11755
Dreyer DR, Jia H-P, Todd AD, Geng J, Bielawski CW (2011) Graphite oxide: a selective and highly efficient oxidant of thiols and sulfides. Org Biomol Chem 9:7292–7295
Marcano DC, Kosynkin DV, Berlin JM, Sinitskii A, Sun Z, Slesarev A, Alemany LB, Lu W, Tour JM (2010) Improved synthesis of graphene oxide. ACS Nano 4:4806–4814
Acknowledgements
Financial support from Science and Engineering Research Board (Grant No EMR/2015/000549), New Delhi, is gratefully acknowledged. PC thanks UGC, New Delhi, for Senior Research Fellowship under UGC–NET.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Article Highlights
• Graphene oxide (GO) catalysis in flow reaction.
• A metal-free continuous-flow synthesis of thioethers.
• The catalytic bed of GO is recyclable for ten cycles.
Electronic supplementary material
ESM 1
(PDF 1899 kb)
Rights and permissions
About this article
Cite this article
Choudhury, P., Basu, B. Graphene oxide-catalyzed two-step continuous-flow conversion of aryl amine to unsymmetrical thioether. J Flow Chem 10, 389–396 (2020). https://doi.org/10.1007/s41981-019-00048-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41981-019-00048-7