Abstract
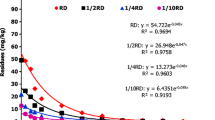
A simple, sensitive and reproducible analytical method for quantitative determination of indoxacarb residues in pigeonpea green pod, pigeonpea dry grain and soil was developed and validated using LC-MS/MS. The developed methodology was linear having correlation coefficients (R2) value of 0.999. The LOD and LOQ were 0.0015 and 0.005 μg g−1, respectively. QuEChERS (Quick, Easy, Cheap, Effective, Rugged and Safe) technique involved for extraction of indoxacarb residues with 1% ethyl acetate in acetonitrile provided acceptable recoveries in the range of 83.80–99.01% at 0.005, 0.025 and 0.050 μg g−1 spiking level with precision RSD of less than 1.20 %. Initial deposit on green pods following two application (15 days interval between application) of indoxacarb 14.5% SC at 60 and 120 g a.i.ha−1 was 0.583 and 1.342 μg g−1 and dissipated to 0.005 and 0.006 μg g−1 on 7 and 10 days after application, respectively. Half-life and safe waiting period (SWP) were ranged from 1.13 to 1.23 days and 3.23 to 5.03 days for the dose 60 and 120 g a.i.ha−1 in pigeonpea green pods, respectively. Hazard index (HI) value was more than 1 in green pods drawn on 5th and 7th day after application in recommended (60 g a.i.ha−1) and double the recommended dose (120 g a.i.ha−1), respectively.




Similar content being viewed by others

References
Allen CT, Kharboutli MS, Capp C Jr, Eearnest L (1999) StewardTM: new insecticides for the new millennium. Special report 193. Arkansas Agricultural Experiment Station, University of Arkansas, Fayetteville, pp 56–64
Anastassiades M, Lehotay SJ, Stajnbaher D, Schenck FJ (2003) Fast and easy multiresidue method employing acetonitrile extraction/partitioning and dispersive solid-phase extraction for the determination of pesticide residues in produce. J AOAC Int 86(2):412–431
Bassi A, Cunsolo D, May L, Parente L, Turchiarelli V, Massasso W, Sandroni D (2000) DPX-MP062 (StewardTM) a new insecticide for IPM in vegetable crops. Efficacy results on Lepidoptera in pepper, tomato, cauliflower and cabbage crops (pp 515–520). In Atti, Giornate Fitopatologiche, Perugia.
Brugger K E (1997) DPX-MP062: prospective tier I ecological effects assessment for non-target organisms. DuPont Agricultural Products Document No. AMR 4782-97.
Darko G, Akoto O (2008) Dietary intake of organophosphorus pesticide residues through vegetables from Kumasi, Ghana. Food Chem Toxicol 46:3703–3706
Dubey OP, Sharma O P (2002) The future of integrated pest management in India. In: Proceedings Integrated Pest Management in Indian Agriculture, NCAP (ICAR), New Delhi (India) and NCIPM, 261-265.
Ebeling W (1963) Analysis of the basic processes involved in the deposition, degradation, persistence, and effectiveness of pesticides. Res Rev 3:35–163
European Commission (2017) SANTE/11813/2017 of 1st January 2016. Guidance document on analytical quality control and method validation procedures for pesticide residues and analysis in food and feed. 1-34.
FSSAI (2018) Notification-pesticide/stds-FSSAI/2017. Food Safety and Standards Authority of India. Ministry of Health and Family Welfare, 24th December, 2018, Available from: file:///C:/Users/HP/Downloads/Gazette_Notification_MRL_Pesticides_03_01_2019.pdf. Accessed 15 Dec.2018.
Gopalan C, Rama Sastri BV, Balasubramanian S C, Revised and updated by Narasinga Rao BS, Deosthale Y G, Pant, KC (1989) Nutritive value of Indian foods. Revised and updated Edition. National Institute of Nutrition, Indian Council of Medical Research, Hyderabad. pp 156.
Gupta S, Sharma RK, Gupta RK, Sinha SR, Gajbhiye VT (2009) Persistence of a new insecticides and their efficacy against insect pests of okra. Bull Environ Contam Toxicol 82:243–247
Hewa-Kapuge S, McDougall S, Hoffman AA (2003) Effects of methoxyfenozide, indoxacarb, and other insecticides on the beneficial egg parasitoid Trichograma brassicae (Hymenoptera: Trichogrammatidae) under laboratory and field conditions. J Econ Entomol 96(4):1083–1090
Lee EY, Kim DK, Park IY, Noh HH, Park YS, Kim TH, Jin CW, Kim KI, Yun SS, Oh SK, Kyung KS (2008) Residue patterns of indoxacarb and thiamethoxam in Chinese cabbage (Brassica campestris L.) grown under greenhouse conditions and their estimated daily intake. Korean J Environ Agric 27:92–98. https://doi.org/10.5338/KJEA.2008.27.1.092
Lehotay SJ, Mastovska K, Yun SJ (2005) Evaluation of two fast and easy methods for pesticide residue analysis in fatty food matrixes. J AOAC Int 88:630–638
Liu TX, Sparks AN Jr (1999) Efficacies of some selected insecticides on diamondback moth and diamondback moth on cabbage in South Texas. Subtrop Plant Sci 51:54–58
McCann SF, Annis GD, Shapiro R, Piotrowshi DW, Lahm GP, Long JK (2001) The discovery of indoxacarb: oxadiazine as a new class of pyrazoline type insecticides. Pest Manag Sci 57(2):153–164
Mohapatra S, Siddamallaiah L, Matadha NY, Udupi VR, Raj DP, Gadigeppa S (2019) Dissipation of neonicotinoid insecticides imidacloprid, indoxacarb and thiamethoxam on pomegranate (Punica granatum L.). Ecotoxicol Environ Saf 17:130–137. https://doi.org/10.1016/j.ecoenv.2018.12.070
Prasanna L, Rao SM, Singh V, Kujur R, Lakshmi P (2008) Indoxacarb poisoning: an unusual presentation as methemoglobinemia. Indian J Crit Care Med 12(4):198–200. https://doi.org/10.4103/0972-5229.45082
Prodhan MDH, Papadakis E, Papadopoulou-Mourkidou E (2016) Variability of pesticide residues in cauliflower units collected from a field trial and market places in Greece. J Environ Sci Health Part B Pestic Food Contam Agric Wastes 51:644–653
Regupathy A, Dhamu KP (2001) Statistics work book for insecticides toxicology, Softeck Computers, Coimbatore, 206.
Sahoo BK, Senapati B (2000) Efficacy and economics of synthetic insecticides and plant products for the control of pod borer incidence in pigeonpea. Indian J Entomol 62:346–352
Sameer Kumar CV, Myer G, Mula SIP, Mula RP, Saxena RK, Rao G, Varshney RK (2014) Pigeonpea perspective in India: in 1st Philippine pigeonpea congress. Mariano Marcos State University, Batac
Santiago da Silva CM, Habermann G, Marchi MRR, Zocolo GJ (2012) The role of matrix effects on the quantification of abscisic acid and its metabolites in the leaves of Bauhinia variegata L. using liquid chromatography combined with tandem mass spectrometry. Braz J Plant Physiol 24(3):223–232. https://doi.org/10.1590/S1677-04202012000300009
Saxena KB, Ravishankar K, Vijaya Kumar R, Sreejith KP, Srivastava RK (2010) Information Bulletin No. 83. Patancheru- 502 324. International Crops Research Institute for the Semi-Arid Tropics, Andhra Pradesh, p 124
Sdeek Fayza A, Taha HS (2018) Indoxacarb residue analysis, dissipation and field efficacy on sugar beet applied for Spodoptera littoralis infestation. Egy Sci J Pestic 4(1):7–12
Shanower TG, Romeis JM, Minja EM (1999) Insect pests of pigeonpea and their management. Annu Rev Entomol 44(1):77–96
Sharma OP, Gopali JB, Yelshetty S, Bambawale OM, Garg DK, Bhosle BB (2010) Pests of pigeonpea and their management. NCIPM, New Delhi
Sinha SR, Gupta RK, Gajbhiye VT, Vishwa N (2010) Bioefficacy and persistence of indoxacarb on Solanum melongena. Ann Plant Prot Sci 18:223–282
Sun DL, Qiu J, Wu YJ, Liang HW, Liu CL, Li L (2012) Enantioselective degradation of indoxacarb in cabbage and soil under field conditions. Chirality 24(8):628–633
Szpyrka E, Matyaszek A, Słowik-Borowiec M (2017) Dissipation of chlorantraniliprole, chlorpyrifos-methyl and Indoxacarb insecticides used to control codling moth (Cydia pomonella L.) and leaf rollers (Tortricidae) in apples for production of baby food. Environ Sci Pollut Res 24:12128–12135
Takkar R, Sahoo SK, Singh G, Mandal K, Battu RS, Singh B (2011) Persistence of indoxacarb on cauliflower (Brassica oleracea var. botrytis. L.) and its risk assessment. Am J Anal Chem 2:69–76
Tiryaki O (2016) Validation of QuEChERS method for the determination of some pesticide residues in two apple varieties. J Environ Sci Health Part B Pestic Food Contam Agric Wastes 51:722–729
United States Environmental Protection Agency (2000) Office of Prevention, Pesticides and Toxic Substances (7505C): Indoxacarb. 2000. Available from: https://www3.epa.gov/pesticides/chem_search/reg_actions/registration/fs_PC-067710_30-Oct-10.pdf. Acessed 31Dec.2018.
Urvashi GJ, Sahoo SK, Kaur S, Battu RS, Singh B (2012) Estimation of indoxacarb residues by QuEChERS technique and its degradation pattern in cabbage. Bull Environ Contam Toxicol 88:372–376. https://doi.org/10.1007/s00128-011-0468-8
Yoon J-Y, Park J-H, Moon H-R, Han G-T, Lee K-S (2013) Residue patterns of indoxacarb and pyridalyl in treated cauliflower. Agric Sci 4(3):111–116. https://doi.org/10.4236/as.2013.43017
Acknowledgements
The authors gratefully acknowledge the University of Agricultural Sciences, Raichur, for field and laboratory research facility support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict(s) of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Statement of Informed Consent
Informed consent is not applicable in this study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Naik, H.R., Pallavi, M.S., Chawan, R. et al. Method Development and Validation for Determination of Indoxacarb Using LC-ESI-MS/MS and Its Dissipation Kinetics in Pigeonpea. Food Anal. Methods 13, 647–657 (2020). https://doi.org/10.1007/s12161-019-01681-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-019-01681-7