Abstract
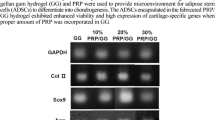
This study describes a protein-based scaffold using platelet rich plasma (PRP), aminated hyaluronic acid (HA-NH2) and Genipin for potential use in regenerative applications as an autologous tissue engineering scaffold. Human PRP was subjected to three freeze–thaw cycles for obtaining platelet lysates (PL). HA-NH2 was synthesized from hyaluronic acid. PL/HA-NH2 scaffolds were fabricated using different concentrations of genipin (0.05, 0.1 and 0.2%) and HA-NH2 (10, 20 and 30 mg/mL). Mechanical, physical, and chemical properties of the scaffolds were comprehensively investigated. The compressive test findings revealed that crosslinking with 0.1 and 0.2% genipin improved the mechanical properties of the scaffolds. SEM evaluations showed that the scaffolds exhibited an interconnected and macroporous structure. Besides, porosimetry analysis indicated a wide distribution of the scaffold pore-size. Rheological findings demonstrated that the G′ values were higher than the G″ values, indicating that PL/HA-NH2 scaffolds had typical viscoelastic properties. In vitro biocompatibility studies showed that the scaffolds were both cytocompatible and hemocompatible. Alamar Blue test indicated that human adipose mesenchymal stem cells (hASCs) were able to attach, spread and proliferate on the scaffolds for 21 days-duration. Our findings clearly indicate that PL/HA-NH2 can be a promising autologous candidate scaffold for tissue engineering applications.








Similar content being viewed by others
References
Kazem-Arki M, Kabiri M, Rad I, Roodbari NH, Hosseinpoor H, Mirzaei S, et al. Enhancement of osteogenic differentiation of adipose-derived stem cells by PRP modified nanofibrous scaffold. Cytotechnology. 2018;70:1487–98.
Sample SJ, Racette MA, Hans EC, Volstad NJ, Schaefer SL, Bleedorn JA, et al. Use of a platelet-rich plasma-collagen scaffold as a bioenhanced repair treatment for management of partial cruciate ligament rupture in dogs. PLoS ONE. 2018;13:e0197204.
Spanò R, Muraglia A, Todeschi MR, Nardini M, Strada P, Cancedda R, et al. Platelet-rich plasma-based bioactive membrane as a new advanced wound care tool. J Tissue Eng Regen Med. 2018;12:e82–96.
Fernandes G, Yang S. Application of platelet-rich plasma with stem cells in bone and periodontal tissue engineering. Bone Res. 2016;4:16036.
Rodrigues AA, Lana JF, Luzo ÂC, Santana MH, Perez AG, Lima-Silva DB, et al. Platelet-rich plasma and tissue engineering. In: Lana JFSD, Santana MHA, Belangero WD, Luzo ACM, editors. Platelet-rich plasma. Berlin, Germany: Springer; 2014. p. 139–151. https://link.springer.com/book/10.1007/978-3-642-40117-6.
Bielecki TM, Gazdzik TS, Arendt J, Szczepanski T, Krol W, Wielkoszynski T. Antibacterial effect of autologous platelet gel enriched with growth factors and other active substances: an in vitro study. J Bone Jt Surg Br. 2007;89:417–20.
Moojen DJF, Everts PA, Schure RM, Overdevest EP, van Zundert A, Knape JT, et al. Antimicrobial activity of platelet-leukocyte gel against Staphylococcus aureus. J Orthop Res. 2008;26:404–10.
Drago L, Bortolin M, Vassena C, Taschieri S, Del Fabbro M. Antimicrobial activity of pure platelet-rich plasma against microorganisms isolated from oral cavity. BMC Microbiol 2013;13:47.
Suthar M, Gupta S, Bukhari S, Ponemone V. Treatment of chronic non-healing ulcers using autologous platelet rich plasma: a case series. J Biomed Sci. 2017;24:16.
Sadeghi-Ataabadi M, Mostafavi-Pour Z, Vojdani Z, Sani M, Latifi M, Talaei-Khozani T. Fabrication and characterization of platelet-rich plasma scaffolds for tissue engineering applications. Mater Sci Eng C Mater Biol Appl. 2017;71:372–80.
Jalowiec JM, D’Este M, Bara JJ, Denom J, Menzel U, Alini M, et al. An in vitro investigation of platelet-rich plasma-gel as a cell and growth factor delivery vehicle for tissue engineering. Tissue Eng Part C Methods. 2016;22:49–58.
Lee HR, Park KM, Joung YK, Park KD, Do SH. Platelet-rich plasma loaded hydrogel scaffold enhances chondrogenic differentiation and maturation with up-regulation of CB1 and CB2. J Control Release. 2012;159:332–7.
Velier M, Magalon J, Daumas A, Cassar M, Francois P, Ghazouane A, et al. Production of platelet-rich plasma gel from elderly patients under antithrombotic drugs: perspectives in chronic wounds care. Platelets. 2018;29:496–503.
Tan H, Li H, Rubin JP, Marra KG. Controlled gelation and degradation rates of injectable hyaluronic acid-based hydrogels through a double crosslinking strategy. J Tissue Eng Regen Med. 2011;5:790–7.
Mero A, Campisi M. Hyaluronic acid bioconjugates for the delivery of bioactive molecules. Polymers. 2014;6:346–69.
Demirdögen B, Elçin AE, Elçin YM. Neovascularization by bFGF releasing hyaluronic acid–gelatin microspheres: in vitro and in vivo studies. Growth Factors. 2010;28:426–36.
Tan H, Marra KG. Injectable, biodegradable hydrogels for tissue engineering applications. Materials. 2010;3:1746–67.
Bulpitt P, Aeschlimann D. New strategy for chemical modification of hyaluronic acid: preparation of functionalized derivatives and their use in the formation of novel biocompatible hydrogels. J Biomed Mater Res. 1999;47:152–69.
Babo P, Santo VE, Duarte ARC, Correia C, Costa MH, Mano JF, et al. Platelet lysate membranes as new autologous templates for tissue engineering applications. Inflamm Regen. 2014;34:033–044.
Parmaksiz M, Dogan A, Odabas S, Elcin AE, Elcin YM. Clinical applications of decellularized extracellular matrices for tissue engineering and regenerative medicine. Biomed Mater. 2016;11:022003.
Elçin YM, Elçin AE, Pappas GD. Functional and morphological characteristics of bovine adrenal chromaffin cells on macroporous poly(D,L-lactide-co-glycolide) scaffolds. Tissue Eng. 2003;9:1047–56.
ISO - 10993-4. Biological evaluation of medical devices. Part 4: Selection of tests for interactions with blood. Geneva: International Organization for Standardization; 1999.
ISO - 10993-5. Biological evaluation of medical devices. Part 5: Tests for cytotoxicity: In vitro methods. Geneva: International Organization for Standardization; 1999.
Elçin AE, Parmaksız M, Doğan A, Şeker Ş, Durkut S, Dalva K, et al. Differential gene expression profiling of human adipose stem cells differentiating into smooth muscle-like cells by TGFb1/BMP4. Exp Cell Res. 2017;352:207–17.
Şeker Ş, Elçin AE, Yumak T, Sınağ A, Elçin YM. In vitro cytotoxicity of hydrothermally synthesized ZnO nanoparticles on human periodontal ligament fibroblast and mouse dermal fibroblast cells. Toxicol Vitr. 2014;28:1349–58.
Sánchez M, Anitua E, Delgado D, Sanchez P, Prado R, Orive G, et al. Platelet-rich plasma, a source of autologous growth factors and biomimetic scaffold for peripheral nerve regeneration. Expert Opin Biol Ther. 2017;17:197–212.
Lee JW, Kwon OH, Kim TK, Cho YK, Choi KY, Chung HY, et al. Platelet-rich plasma: quantitative assessment of growth factor levels and comparative analysis of activated and inactivated groups. Arch Plast Surg. 2013;40:530–5.
Lohmann M, Walenda G, Hemeda H, Joussen S, Drescher W, Jockenhoevel S, et al. Donor age of human platelet lysate affects proliferation and differentiation of mesenchymal stem cells. PLoS ONE 2012;7:e37839.
Wang Z, Wang Z, Lu WW, Zhen W, Yang D, Peng S. Novel biomaterial strategies for controlled growth factor delivery for biomedical applications. NPG Asia Mater. 2017;9:e435.
Moura MJ, Faneca H, Lima MP, Gil MH, Figueiredo MM. In situ forming chitosan hydrogels prepared via ionic/covalent co-cross-linking. Biomacromolecules. 2011;12:3275–84.
Massensini AR, Ghuman H, Saldin LT, Medberry CJ, Keane TJ, Nicholls FJ, et al. Concentration-dependent rheological properties of ECM hydrogel for intracerebral delivery to a stroke cavity. Acta Biomater. 2015;27:116–30.
Perazzo A, Nunes JK, Guido S, Stone HA. Flow-induced gelation of microfiber suspensions. Proc Natl Acad Sci USA. 2017;114:E8557–64.
Sundararaghavan HG, Monteiro GA, Lapin NA, Chabal YJ, Miksan JR, Shreiber DI. Genipin-induced changes in collagen gels: Correlation of mechanical properties to fluorescence. J Biomed Mater Res A. 2008;87:308–20.
Xu B, Chow MJ, Zhang Y. Experimental and modeling study of collagen scaffolds with the effects of crosslinking and fiber alignment. Int J Biomater. 2011;2011:172389.
Aramwit P, Siritientong T, Kanokpanont S, Srichana T. Formulation and characterization of silk sericin–PVA scaffold crosslinked with genipin. Int J Biol Macromol. 2010;47:668–75.
Wang C, Wang S, Li K, Ju Y, Li J, Zhang Y, et al. Preparation of laponite bioceramics for potential bone tissue engineering applications. PLoS ONE. 2014;9:e99585.
Gao J, Guo H, Zhao L, Zhao X, Wang L. Water-stability and biological behavior of electrospun collagen/PEO fibers by environmental friendly crosslinking. Fiber Polym. 2017;18:1496–503.
Peng Z, Peng Z, Shen Y. Study on biological safety of polyvinyl alcohol/collagen hydrogel as a tissue substitute (II). J Macromol Sci A. 2011;48:632–6.
Alehosseini M, Golafshan N, Kharaziha M, Fathi M, Edris H. Hemocompatible and bioactive heparin-loaded PCL-α-TCP fibrous membranes for bone tissue engineering. Macromol Biosci 2018;18:1800020.
Zhao M, Cao Y, Liu X, Deng J, Li D, Gu H. Effect of nitrogen atomic percentage on N+-bombarded MWCNTs in cytocompatibility and hemocompatibility. Nanoscale Res Lett. 2014;9:142.
Minh HH, Hiep NT, Hai ND, Toi VV. Fabrication of polycaprolactone/polyurethane loading conjugated linoleic acid and its antiplatelet adhesion. Int J Biomater. 2017;2017:5690625.
Haugh MG, Murphy CM, McKiernan RC, Altenbuchner C, O’Brien FJ. Crosslinking and mechanical properties significantly influence cell attachment, proliferation, and migration within collagen glycosaminoglycan scaffolds. Tissue Eng Part A. 2011;17:1201–8.
Robinson ST, Douglas AM, Chadid T, Kuo K, Rajabalan A, Li H, et al. A novel platelet lysate hydrogel for endothelial cell and mesenchymal stem cell-directed neovascularization. Acta Biomater. 2016;36:86–98.
Moroz A, Bittencourt RAC, Almeida RP, Felisbino SL, Deffune E. Platelet lysate 3D scaffold supports mesenchymal stem cell chondrogenesis: an improved approach in cartilage tissue engineering. Platelets. 2013;24:219–25.
Costa-Almeida R, Franco AR, Pesqueira T, Oliveira MB, Babo PS, Leonor IB, et al. The effects of platelet lysate patches on the activity of tendon-derived cells. Acta Biomater. 2018;68:29–40.
Acknowledgements
This work was financially supported by the Scientific and Technological Research Council of Turkey (TUBİTAK) [Grant Number 216M424].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
YME is the founder and shareholder of Biovalda Health Technologies, Inc. (Ankara, Turkey). The authors have patent applications in relation to regenerative biomaterials. The authors declare no competing financial interests in relation to this particular article. The authors are alone responsible for the content and writing of the paper.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Şeker, Ş., Elçin, A.E. & Elçin, Y.M. Autologous protein-based scaffold composed of platelet lysate and aminated hyaluronic acid. J Mater Sci: Mater Med 30, 127 (2019). https://doi.org/10.1007/s10856-019-6334-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10856-019-6334-7