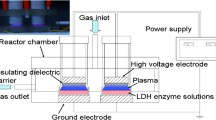
Abstract
Effects of atmospheric pressure plasmas on proteins are studied to assess the quality of plasma decontamination and to gain insights into plasma-triggered molecular events underlying observations made in plasma medicine on the cellular, organ, and systemic level. Atmospheric pressure plasma treatment has been reported to cause protein degradation. Degradation products, however, have not been characterized. Treating different model proteins in aqueous solution with a DBD plasma, we confirmed with different methods (Bradford assay, application of Lambert–Beer’s law on absorption measurements at 280 nm, ninhydrin assay, size exclusion chromatography, SDS-PAGE) that protein degradation takes place. Peptides of different sizes were detected by size exclusion chromatography. The ninhydrin assay indicated that peptide bonds are cleaved. In the presence of hydroxyl radical scavenger d-mannitol, the concentration of amino termini formed during the initial 10 min of plasma treatment was reduced by 96%, while at longer treatment times mannitol did no longer prevent the formation of amino termini, indicating that hydroxyl radicals play an important role in the initial cleaving of peptide bonds in the protein, but other mechanisms are at play in cleaving the peptide bonds in the resulting peptides. The generation of peptides has implications for plasma decontamination and plasma medicine. It is critical to verify that plasma decontamination processes do not result in protein fragments with undesired properties. In plasma medicine, plasma-generated protein fragments may act as molecular triggers in treated cells, tissues, or patients, e.g., regulating signaling cascades in a protease-like fashion.






Similar content being viewed by others
References
Sakiyama Y, Graves DB, Chang H-W et al (2012) Plasma chemistry model of surface microdischarge in humid air and dynamics of reactive neutral species. J Phys D Appl Phys 45:425201. https://doi.org/10.1088/0022-3727/45/42/425201
Haertel B, Woedtke TV, Weltmann K-D et al (2014) Non-thermal atmospheric-pressure plasma possible application in wound healing. Biomol Ther 22:477. https://doi.org/10.4062/biomolther.2014.105
Moreau M, Orange N, Feuilloley MGJ (2008) Non-thermal plasma technologies: new tools for bio-decontamination. Biotechnol Adv 26:610. https://doi.org/10.1016/j.biotechadv.2008.08.001
Laroussi M (2005) Low temperature plasma-based sterilization: overview and state-of-the-art. Plasma Process Polym 2:391. https://doi.org/10.1002/ppap.200400078
Julák J, Janoušková O, Scholtz V et al (2011) Inactivation of prions using electrical dc discharges at atmospheric pressure and ambient temperature. Plasma Process Polym 8:316. https://doi.org/10.1002/ppap.201000100
Baxter HC, Campbell GA, Whittaker AG et al (2005) Elimination of transmissible spongiform encephalopathy infectivity and decontamination of surgical instruments by using radio-frequency gas-plasma treatment. J Gen Virol 86:2393. https://doi.org/10.1099/vir.0.81016-0
Lackmann J-W, Schneider S, Edengeiser E et al (2013) Photons and particles emitted from cold atmospheric-pressure plasma inactivate bacteria and biomolecules independently and synergistically. J R Soc Interface 10:20130591. https://doi.org/10.1098/rsif.2013.0591
Rauscher H, Stapelmann K, Kylián O et al (2009) Monitoring plasma etching of biomolecules by imaging ellipsometry. Vacuum 84:75. https://doi.org/10.1016/j.vacuum.2009.05.012
Takai E, Kitano K, Kuwabara J et al (2012) Protein inactivation by low-temperature atmospheric pressure plasma in aqueous solution. Plasma Process Polym 9:77. https://doi.org/10.1002/ppap.201100063
Lee HJ, Shon CH, Kim YS et al (2009) Degradation of adhesion molecules of G361 melanoma cells by a non-thermal atmospheric pressure microplasma. New J Phys 11:115026. https://doi.org/10.1088/1367-2630/11/11/115026
Deng XT, Shi JJ, Kong MG (2007) Protein destruction by a helium atmospheric pressure glow discharge: capability and mechanisms. J Appl Phys 101:074701. https://doi.org/10.1063/1.2717576
De Backer J, Razzokov J, Hammerschmid D et al (2018) The effect of reactive oxygen and nitrogen species on the structure of cytoglobin: a potential tumor suppressor. Redox Biol 19:1. https://doi.org/10.1016/j.redox.2018.07.019
Brown DR, Schmidt B, Kretzschmar HA (1996) Role of microglia and host prion protein in neurotoxicity of a prion protein fragment. Nature 380:345
Stringfellow HM, Jones MR, Green MC et al (2014) Selectivity in ROS-induced peptide backbone bond cleavage. J Phys Chem A 118:11399. https://doi.org/10.1021/jp508877m
Platis IE, Ermacora MR, Fox RO (1993) Oxidative polypeptide cleavage mediated by EDTA-iron covalently linked to cysteine residues. Biochemistry 32:12761
Berlett BS, Stadtman ER (1997) Protein oxidation in aging, disease, and oxidative stress. J Biol Chem 272:20313. https://doi.org/10.1074/jbc.272.33.20313
Kuchenbecker M, Bibinov N, Kaemlimg A et al (2009) Characterization of DBD plasma source for biomedical applications. J Phys D Appl Phys 42:045212
Kitagawa M, Ara T, Arifuzzaman M et al (2005) Complete set of ORF clones of Escherichia coli ASKA library (a complete set of E. coli K-12 ORF archive): unique resources for biological research. DNA Res 12:291. https://doi.org/10.1093/dnares/dsi012
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248
Sambrook J, Russell DW (2001) Molecular cloning: a laboratory manual, 3rd edn. Cold Spring Harbor Laboratory Press, New York
Friedman M (2004) Applications of the ninhydrin reaction for analysis of amino acids, peptides, and proteins to agricultural and biomedical sciences. J Agric Food Chem 52:385. https://doi.org/10.1021/jf030490p
Girard F, Badets V, Blanc S et al (2016) Formation of reactive nitrogen species including peroxynitrite in physiological buffer exposed to cold atmospheric plasma. RSC Adv 6:78457. https://doi.org/10.1039/c6ra12791f
Krewing M, Stepanek JJ, Cremers C et al (2019) The molecular chaperone Hsp33 is activated by atmospheric-pressure plasma protecting proteins from aggregation. J R Soc Interface 16:20180966. https://doi.org/10.1098/rsif.2018.0966
Lackmann J-W, Baldus S, Steinborn E et al (2015) A dielectric barrier discharge terminally inactivates RNase A by oxidizing sulfur-containing amino acids and breaking structural disulfide bonds. J Phys D Appl Phys 48:494003. https://doi.org/10.1088/0022-3727/48/49/494003
Chauvin J, Judée F, Yousfi M et al (2017) Analysis of reactive oxygen and nitrogen species generated in three liquid media by low temperature helium plasma jet. Sci Rep. https://doi.org/10.1038/s41598-017-04650-4
Arjunan KP, Clyne AM (2011) Hydroxyl radical and hydrogen peroxide are primarily responsible for dielectric barrier discharge plasma-induced angiogenesis. Plasma Process Polym 8:1154. https://doi.org/10.1002/ppap.201100078
Takai E, Kitamura T, Kuwabara J et al (2014) Chemical modification of amino acids by atmospheric-pressure cold plasma in aqueous solution. J Phys D Appl Phys 47:285403. https://doi.org/10.1088/0022-3727/47/28/285403
Bruggeman PJ, Kushner MJ, Locke BR et al (2016) Plasma–liquid interactions: a review and roadmap. Plasma Sources Sci Technol 25:053002
Balzer J, Demir E, Kogelheide F et al (2019) Cold atmospheric plasma (CAP) differently affects migration and differentiation of keratinocytes via hydrogen peroxide and nitric oxide-related products. Clin Plasma Med 13:1. https://doi.org/10.1016/j.cpme.2018.11.001
Goldstein S, Czapski G (1984) Mannitol as an OH· scavenger in aqueous solutions and in biological systems. Int J Radiat Biol Relat Stud Phys Chem Med 46:725. https://doi.org/10.1080/09553008414551961
Baldus S, Schröder D, Bibinov N et al (2015) Atomic oxygen dynamics in an air dielectric barrier discharge: a combined diagnostic and modeling approach. J Phys D Appl Phys 48:275203. https://doi.org/10.1088/0022-3727/48/27/275203
Bauer G, Graves DB (2016) Mechanisms of selective antitumor action of cold atmospheric plasma-derived reactive oxygen and nitrogen species. Plasma Process Polym 13:1157. https://doi.org/10.1002/ppap.201600089
Liu ZW, Yang XF, Zhu AM et al (2008) Determination of the OH radical in atmospheric pressure dielectric barrier discharge plasmas using near infrared cavity ring-down spectroscopy. Eur Phys J D 48:365. https://doi.org/10.1140/epjd/e2008-00110-7
Hibert C, Gaurand I, Motret O et al (1999) [OH (X)] measurements by resonant absorption spectroscopy in a pulsed dielectric barrier discharge. J Appl Phys 85:7070
Ono R, Oda T (2002) Dynamics and density estimation of hydroxyl radicals in a pulsed corona discharge. J Phys D Appl Phys 35:2133
Keyer K, Imlay JA (1996) Superoxide accelerates DNA damage by elevating free-iron levels. Proc Natl Acad Sci U S A 93:13635. https://doi.org/10.1073/pnas.93.24.13635
Acknowledgements
We thank Abdulkadir Yayci, Franz Narberhaus, and Lars Leichert for the fruitful discussions on this topic and Cinogy for providing the DBD source.
Funding
JEB gratefully acknowledges funding from the German Research Foundation (BA 4193/7-1 and CRC1316).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Krewing, M., Schubert, B. & Bandow, J.E. A Dielectric Barrier Discharge Plasma Degrades Proteins to Peptides by Cleaving the Peptide Bond. Plasma Chem Plasma Process 40, 685–696 (2020). https://doi.org/10.1007/s11090-019-10053-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11090-019-10053-2