Abstract
The aim of the present study was to examine the neuroprotective potential of pioglitazone via activation of Nrf2/ARE-dependent HO-1 signaling pathway in chronic neuroinflammation and progressive neurodegeneration mouse model induced by lipopolysaccharide (LPS). After assessing spatial memory, anxiety and motor-coordination, TH+ neurons in substantia nigra (SN) were counted. The oxidative stress marker carbonyl protein levels and HO-1 enzyme activity were also evaluated. RT-qPCR was conducted to detect HO-1, Nrf2 and NF-κp65 mRNA expression levels and Nrf2 transcriptional activation of antioxidant response element (ARE) of HO-1 was investigated. Pioglitazone ameliorated LPS-induced dopaminergic neuronal loss, as well as mitigated neurobehavioral impairments. It enhanced Nrf2 mRNA expression, and augmented Nrf2/ARE-dependent HO-1 pathway activation by amplifying HO-1 mRNA expression. Moreover, it induced a significant decrease in NF-κB p65 mRNA expression, while reducing carbonyl protein levels and restoring the HO-1 enzyme activity. Interestingly, LPS induced Nrf2/antioxidant response element (ARE) of HO-1 activation, ultimately resulting in slight enhanced HO-1 mRNA expression. However, LPS elicited decrease in HO-1 enzyme activity. Zinc protoporphyrin-IX (ZnPPIX) administrated with pioglitazone abolished its effects in the LPS mouse model. The study results demonstrate that coordinated activation of Nrf2/ARE-dependent HO-1 pathway defense mechanism by the PPARγ agonist pioglitazone mediated its neuroprotective effects.





Similar content being viewed by others
References
Niranjan R (2014) The Role of inflammatory and oxidative stress mechanisms in the pathogenesis of Parkinson’s disease: focus on astrocytes. Mol Neurobiol 49:28–38. https://doi.org/10.1007/s12035-013-8483-x
Fan K, Wu X, Fan B et al (2012) Up-regulation of microglial cathepsin C expression and activity in lipopolysaccharide-induced neuroinflammation. J Neuroinflamm 9:96. https://doi.org/10.1186/1742-2094-9-96
Fan K, Li D, Zhang Y et al (2015) The induction of neuronal death by up-regulated microglial cathepsin H in LPS-induced neuroinflammation. J Neuroinflamm 12:54. https://doi.org/10.1186/s12974-015-0268-x
Qin L, Wu X, Block ML et al (2007) Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia 55:453–462. https://doi.org/10.1002/glia.20467
Reinert KRS, Umphlet CD, Quattlebaum A, Boger HA (2014) Short-term effects of an endotoxin on substantia nigra dopamine neurons. Brain Res 1557:164–170. https://doi.org/10.1016/j.brainres.2014.02.005
Wu SY, Wang TF, Yu L et al (2011) Running exercise protects the substantia nigra dopaminergic neurons against inflammation-induced degeneration via the activation of BDNF signaling pathway. Brain Behav Immun 25:135–146. https://doi.org/10.1016/j.bbi.2010.09.006
Beier EE, Neal M, Alam G et al (2017) Alternative microglial activation is associated with cessation of progressive dopamine neuron loss in mice systemically administered lipopolysaccharide. Neurobiol Dis 108:115–127. https://doi.org/10.1016/j.nbd.2017.08.009
Prakash A, Kumar A, Ming LC et al (2015) Modulation of the nitrergic pathway via activation of PPAR-γ contributes to the neuroprotective effect of pioglitazone against streptozotocin-induced memory dysfunction. J Mol Neurosci 56:739–750. https://doi.org/10.1007/s12031-015-0508-7
Pilipović K, Župan Ž, Dolenec P et al (2015) A single dose of PPARγ agonist pioglitazone reduces cortical oxidative damage and microglial reaction following lateral fluid percussion brain injury in rats. Prog Neuropsychopharmacol Biol Psychiatry 59:8–20. https://doi.org/10.1016/j.pnpbp.2015.01.003
Fiander MDJ, Stifani N, Nichols M et al (2017) Kinematic gait parameters are highly sensitive measures of motor deficits and spinal cord injury in mice subjected to experimental autoimmune encephalomyelitis. Behav Brain Res 317:95–108. https://doi.org/10.1016/j.bbr.2016.09.034
Barbiero JK, Santiago RM, Persike DS et al (2014) Neuroprotective effects of peroxisome proliferator-activated receptor alpha and gamma agonists in model of parkinsonism induced by intranigral 1-methyl-4-phenyl-1,2,3,6-tetrahyropyridine. Behav Brain Res 274:390–399. https://doi.org/10.1016/j.bbr.2014.08.014
Kumar P, Kaundal RK, More S, Sharma SS (2009) Beneficial effects of pioglitazone on cognitive impairment in MPTP model of Parkinson’s disease. Behav Brain Res 197:398–403. https://doi.org/10.1016/j.bbr.2008.10.010
Swanson CR, Joers V, Bondarenko V et al (2011) The PPAR-γ agonist pioglitazone modulates inflammation and induces neuroprotection in parkinsonian monkeys. J Neuroinflamm 8:91. https://doi.org/10.1186/1742-2094-8-91
Iranpour N, Zandifar A, Farokhnia M et al (2016) The effects of pioglitazone adjuvant therapy on negative symptoms of patients with chronic schizophrenia: a double-blind and placebo-controlled trial. Hum Psychopharmacol 31:103–112. https://doi.org/10.1002/hup.2517
Heneka MT, Fink A, Doblhammer G (2015) Effect of pioglitazone medication on the incidence of dementia. Ann Neurol 78:284–294. https://doi.org/10.1002/ana.24439
Brakedal B, Flønes I, Reiter SF et al (2017) Glitazone use associated with reduced risk of Parkinson’s disease. Mov Disord 32:1594–1599. https://doi.org/10.1002/mds.27128
Brauer R, Bhaskaran K, Chaturvedi N et al (2015) Glitazone treatment and incidence of Parkinson’s disease among people with diabetes: a retrospective cohort study. PLoS Med 12:e1001854. https://doi.org/10.1371/journal.pmed.1001854
Cummings J, Morstorf T, Lee G (2016) Alzheimer’s drug-development pipeline: 2016. Alzheimer’s Dement 2:222–232. https://doi.org/10.1016/j.trci.2016.07.001
NINDS Exploratory Trials in Parkinson Disease (NET-PD) FS-ZONE Investigators (2015) Pioglitazone in early Parkinson’s disease: a phase 2, multicentre, double-blind, randomised trial. Lancet Neurol 14:795–803. https://doi.org/10.1016/S1474-4422(15)00144-1
Chao X-J, Chen Z-W, Liu A-M et al (2014) Effect of tacrine-3-caffeic Acid, a novel multifunctional anti-Alzheimer’s dimer, against oxidative-stress-induced cell death in HT22 hippocampal neurons: involvement of Nrf2/HO-1 pathway. CNS Neurosci Ther 20:840–850. https://doi.org/10.1111/cns.12286
Piras S, Furfaro AL, Brondolo L et al (2017) Differentiation impairs Bach1 dependent HO-1 activation and increases sensitivity to oxidative stress in SH-SY5Y neuroblastoma cells. Sci Rep 7:7568. https://doi.org/10.1038/s41598-017-08095-7
Ryter SW, Choi AMK (2002) Heme oxygenase-1: molecular mechanisms of gene expression in oxygen-related stress. Antioxid Redox Signal 4:625–632. https://doi.org/10.1089/15230860260220120
Hung S-Y, Liou H-C, Fu W-M (2010) The mechanism of heme oxygenase-1 action involved in the enhancement of neurotrophic factor expression. Neuropharmacology 58:321–329. https://doi.org/10.1016/j.neuropharm.2009.11.003
Cho H-S, Kim S, Lee S-Y et al (2008) Protective effect of the green tea component, l-theanine on environmental toxins-induced neuronal cell death. NeuroToxicology 29:656–662. https://doi.org/10.1016/j.neuro.2008.03.004
Sheng X-J, Tu H-J, Chien W-L et al (2017) Antagonism of proteasome inhibitor-induced heme oxygenase-1 expression by PINK1 mutation. PLoS ONE 12:e0183076. https://doi.org/10.1371/journal.pone.0183076
He Q, Song N, Jia F et al (2013) Role of α-synuclein aggregation and the nuclear factor E2-related factor 2/heme oxygenase-1 pathway in iron-induced neurotoxicity. Int J Biochem Cell Biol 45:1019–1030. https://doi.org/10.1016/j.biocel.2013.02.012
Mateo I, Infante J, Sánchez-Juan P et al (2010) Serum heme oxygenase-1 levels are increased in Parkinson’s disease but not in Alzheimer’s disease. Acta Neurol Scand 121:136–138. https://doi.org/10.1111/j.1600-0404.2009.01261.x
Lastres-Becker I, Ulusoy A, Innamorato NG et al (2012) α-Synuclein expression and Nrf2 deficiency cooperate to aggravate protein aggregation, neuronal death and inflammation in early-stage Parkinson’s disease. Hum Mol Genet 21:3173–3192. https://doi.org/10.1093/hmg/dds143
Wang G, Liu L, Zhang Y et al (2014) Activation of PPARγ attenuates LPS-induced acute lung injury by inhibition of HMGB1-RAGE levels. Eur J Pharmacol 726:27–32. https://doi.org/10.1016/j.ejphar.2014.01.030
Park SY, Bae JU, Hong KW, Kim CD (2011) HO-1 induced by cilostazol protects against TNF-α-associated cytotoxicity via a PPAR-γ-dependent pathway in human endothelial cells. Korean J Physiol Pharmacol 15:83. https://doi.org/10.4196/kjpp.2011.15.2.83
Cho RL, Yang CC, Tseng HC et al (2018) Haem oxygenase-1 up-regulation by rosiglitazone via ROS-dependent Nrf2-antioxidant response elements axis or PPARγ attenuates LPS-mediated lung inflammation. Br J Pharmacol 175:3928–3946. https://doi.org/10.1111/bph.14465
Park EJ, Jang HJ, Tsoyi K et al (2013) The heme oxygenase-1 inducer THI-56 negatively regulates iNOS expression and HMGB1 release in LPS-activated RAW 264.7 cells and CLP-induced septic mice. PLoS ONE 8:e76293. https://doi.org/10.1371/journal.pone.0076293
Jing X, Wei X, Ren M et al (2016) Neuroprotective effects of tanshinone I against 6-OHDA-induced oxidative stress in cellular and mouse model of Parkinson’s disease through upregulating Nrf2. Neurochem Res 41:779–786. https://doi.org/10.1007/s11064-015-1751-6
Lastres-Becker I, García-Yagüe AJ, Scannevin RH et al (2016) Repurposing the NRF2 activator dimethyl fumarate as therapy against synucleinopathy in Parkinson’s disease. Antioxid Redox Signal 25:61–77. https://doi.org/10.1089/ars.2015.6549
Ramsey CP, Glass CA, Montgomery MB et al (2007) Expression of Nrf2 in neurodegenerative diseases. J Neuropathol Exp Neurol 66:75–85. https://doi.org/10.1097/nen.0b013e31802d6da9
Polvani S, Tarocchi M, Galli A (2012) PPAR and oxidative stress: con() catenating NRF2 and FOXO. PPAR Res 2012:1–15. https://doi.org/10.1155/2012/641087
Zhao XR, Gonzales N, Aronowski J (2015) Pleiotropic role of PPARγ in intracerebral hemorrhage: an intricate system involving Nrf2, RXR and NF-κB. CNS Neurosci Ther 21:357–366. https://doi.org/10.1111/cns.12350
Corona JC, de Souza SC, Duchen MR (2014) PPARγ activation rescues mitochondrial function from inhibition of complex I and loss of PINK1. Exp Neurol 253:16–27. https://doi.org/10.1016/j.expneurol.2013.12.012
Li Q, Tian Z, Wang M et al (2019) Luteoloside attenuates neuroinflammation in focal cerebral ischemia in rats via regulation of the PPARγ/Nrf2/NF-κB signaling pathway. Int Immunopharmacol 66:309–316. https://doi.org/10.1016/J.INTIMP.2018.11.044
Armagan G, Sevgili E, Gürkan FT et al (2019) Regulation of the Nrf2 pathway by glycogen synthase kinase-3β in MPP+-induced cell damage. Molecules. https://doi.org/10.3390/molecules24071377
Ji M, Tang H, Luo D et al (2017) Environmental conditions differentially affect neurobehavioral outcomes in a mouse model of sepsis-associated encephalopathy. Oncotarget 8:82376–82389. https://doi.org/10.18632/oncotarget.19595
Kurauchi Y, Hisatsune A, Isohama Y et al (2012) Caffeic acid phenethyl ester protects nigral dopaminergic neurons via dual mechanisms involving haem oxygenase-1 and brain-derived neurotrophic. Br J Pharmacol 166:1151–1168. https://doi.org/10.1111/j.1476-5381.2012.01833.x
Pan H, He M, Liu R et al (2014) Sulforaphane protects rodent retinas against ischemia-reperfusion injury through the activation of the Nrf2/HO-1 antioxidant pathway. PLoS ONE 9:e114186. https://doi.org/10.1371/journal.pone.0114186
Yang Y, Li X, Zhang L et al (2015) Ginsenoside Rg1 suppressed inflammation and neuron apoptosis by activating PPARγ/HO-1 in hippocampus in rat model of cerebral ischemia-reperfusion injury. Int J Clin Exp Pathol 8:2484–2494
Pérez-de-Puig I, Martín A, Gorina R et al (2013) Induction of hemeoxygenase-1 expression after inhibition of hemeoxygenase activity promotes inflammation and worsens ischemic brain damage in mice. Neuroscience 243:22–32. https://doi.org/10.1016/j.neuroscience.2013.03.046
Ouyang Y, Li D, Wang H et al (2019) MiR-21-5p/dual-specificity phosphatase 8 signalling mediates the anti-inflammatory effect of haem oxygenase-1 in aged intracerebral haemorrhage rats. Aging Cell. https://doi.org/10.1111/acel.13022
Tronel C, Rochefort GY, Arlicot N et al (2013) Oxidative stress is related to the deleterious effects of heme oxygenase-1 in an in vivo neuroinflammatory rat model. Oxid Med Cell Longev. https://doi.org/10.1155/2013/264935
Wan J, Gong X, Jiang R et al (2013) Antipyretic and anti-inflammatory effects of asiaticoside in lipopolysaccharide-treated rat through up-regulation of heme oxygenase-1. Phytother Res 27:1136–1142. https://doi.org/10.1002/ptr.4838
Ghosh A, Birngruber T, Sattler W et al (2014) Assessment of blood–brain barrier function and the neuroinflammatory response in the rat brain by using cerebral open flow microperfusion (cOFM). PLoS ONE 9:e98143. https://doi.org/10.1371/journal.pone.0098143
Wang C-Y, Wang Z-Y, Xie J-W et al (2016) Dl-3-n-butylphthalide-induced upregulation of antioxidant defense is involved in the enhancement of cross talk between CREB and Nrf2 in an Alzheimer’s disease mouse model. Neurobiol Aging 38:32–46. https://doi.org/10.1016/j.neurobiolaging.2015.10.024
André C, Dinel AL, Ferreira G et al (2014) Diet-induced obesity progressively alters cognition, anxiety-like behavior and lipopolysaccharide-induced depressive-like behavior: focus on brain indoleamine 2,3-dioxygenase activation. Brain Behav Immun. https://doi.org/10.1016/j.bbi.2014.03.012
Metz GA, Whishaw IQ (2009) The ladder rung walking task: a scoring system and its practical application. J Vis Exp. https://doi.org/10.3791/1204
Nie S, Xu Y, Chen G et al (2015) Small molecule TrkB agonist deoxygedunin protects nigrostriatal dopaminergic neurons from 6-OHDA and MPTP induced neurotoxicity in rodents. Neuropharmacology 99:448–458. https://doi.org/10.1016/J.NEUROPHARM.2015.08.016
Shih R-H, Cheng S-E, Tung W-H, Yang C-M (2010) Up-regulation of heme oxygenase-1 protects against cold injury-induced brain damage: a laboratory-based study. J Neurotrauma 27:1477–1487. https://doi.org/10.1089/neu.2009.1201
Schmittgen TD, Jiang J, Liu Q, Yang L (2004) A high-throughput method to monitor the expression of microRNA precursors. Nucleic Acids Res 32:43e–43. https://doi.org/10.1093/nar/gnh040
Dalle-Donne I, Aldini G, Carini M et al (2006) Protein carbonylation, cellular dysfunction, and disease progression. J Cell Mol Med 10:389–406. https://doi.org/10.1111/j.1582-4934.2006.tb00407.x
Müllebner A, Moldzio R, Redl H et al (2015) Heme degradation by heme oxygenase protects mitochondria but induces ER stress via formed bilirubin. Biomolecules 5:679–701. https://doi.org/10.3390/biom5020679
Cho H-S, Shin M-S, Song W et al (2013) Treadmill exercise alleviates short-term memory impairment in 6-hydroxydopamine-induced Parkinson’s rats. J Exerc Rehabil 9:354–361. https://doi.org/10.12965/jer.130048
Anderson ST, Commins S, Moynagh PN, Coogan AN (2015) Lipopolysaccharide-induced sepsis induces long-lasting affective changes in the mouse. Brain Behav Immun 43:98–109. https://doi.org/10.1016/j.bbi.2014.07.007
Li D, Song T, Yang L et al (2016) Neuroprotective actions of pterostilbene on hypoxic-ischemic brain damage in neonatal rats through upregulation of heme oxygenase-1. Int J Dev Neurosci 54:22–31. https://doi.org/10.1016/J.IJDEVNEU.2016.08.005
Venkateshappa C, Harish G, Mythri RB et al (2012) Increased oxidative damage and decreased antioxidant function in aging human substantia nigra compared to striatum: implications for Parkinson’s disease. Neurochem Res 37:358–369. https://doi.org/10.1007/s11064-011-0619-7
Colín-González AL, Orozco-Ibarra M, Chánez-Cárdenas ME et al (2013) Heme oxygenase-1 (HO-1) upregulation delays morphological and oxidative damage induced in an excitotoxic/pro-oxidant model in the rat striatum. Neuroscience 231:91–101. https://doi.org/10.1016/j.neuroscience.2012.11.031
Hunter RL, Dragicevic N, Seifert K et al (2007) Inflammation induces mitochondrial dysfunction and dopaminergic neurodegeneration in the nigrostriatal system. J Neurochem 100:1375–1386. https://doi.org/10.1111/j.1471-4159.2006.04327.x
Hong JM, Lee SM (2018) Heme oxygenase-1 protects liver against ischemia/reperfusion injury via phosphoglycerate mutase family member 5-mediated mitochondrial quality control. Life Sci 200:94–104. https://doi.org/10.1016/j.lfs.2018.03.017
Schulz S, Wong RJ, Vreman HJ, Stevenson DK (2012) Metalloporphyrins—an update. Front Pharmacol 3:68. https://doi.org/10.3389/fphar.2012.00068
Fan J, Xu G, Jiang T, Qin Y (2012) Pharmacologic induction of heme oxygenase-1 plays a protective role in diabetic retinopathy in rats. Invest Ophthalmol Vis Sci 53:6541–6556. https://doi.org/10.1167/iovs.11-9241
Choi MJ, Lee EJ, Park JS et al (2017) Anti-inflammatory mechanism of galangin in lipopolysaccharide-stimulated microglia: critical role of PPAR-γ signaling pathway. Biochem Pharmacol 144:120–131. https://doi.org/10.1016/j.bcp.2017.07.021
Lin C-C, Yang C-C, Chen Y-W et al (2018) Arachidonic Acid induces ARE/Nrf2-dependent heme oxygenase-1 transcription in rat brain astrocytes. Mol Neurobiol 55:3328–3343. https://doi.org/10.1007/s12035-017-0590-7
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
AZ conceived of the presented idea, preformed the experimental work and wrote the manuscript; MR contributed in the experimental work; LM co- supervised the study; KAA contributed in the experimental work, conceived the presented idea and supervised the study. All the authors discussed the results and contributed in the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be constructed as a potential conflict of interest.
Ethics Approval
This study was carried out in accordance with the principles and recommendations of the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The protocol was approved by the ethics committee of the German University in Cairo.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 1 (TIFF 2350 kb)
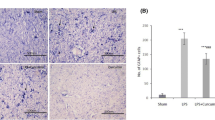
Fig. 1: Histology of H&E stained substantia nigra. Reduction in neurons, and increase in astrocytic gliosis in LPS and ZnPPIX-injected mice, these findings were attenuated with administration of pioglitazone for 7 days (scale bar: 20 µm)
Rights and permissions
About this article
Cite this article
Zakaria, A., Rady, M., Mahran, L. et al. Pioglitazone Attenuates Lipopolysaccharide-Induced Oxidative Stress, Dopaminergic Neuronal Loss and Neurobehavioral Impairment by Activating Nrf2/ARE/HO-1. Neurochem Res 44, 2856–2868 (2019). https://doi.org/10.1007/s11064-019-02907-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-019-02907-0