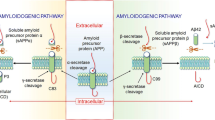
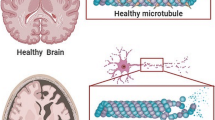
Abstract
Alzheimer’s disease (AD) is the main cause of dementia in elderly. Increasing life expectancy is behind the growing prevalence of AD worldwide with approximately 45 million cases currently documented and projection studies suggesting a triplication of this number by 2050. Mexico does not have an accurate AD registry, but 860,000 cases were reported in 2014 and the prediction reaches 3.5 million cases by 2050. Amyloid plaques and neurofibrillary tangles represent the main hallmarks of AD, being constituted of amyloid beta (Aβ) peptide and phosphorylated tau, respectively. The risk factors for AD include genetic mutations, lifestyle and environmental pollution. Particularly, lead (Pb) has attracted attention due to its ability to target multiple pathways involved in the pathophysiology of AD. Although the epidemiological data are limiting, animal and in vitro studies show growing evidence of causal effects of Pb exposure on AD-linked features including Aβ aggregation and tau phosphorylation. Interestingly, many Pb effects occur selectively following early-life exposure to the metal, suggesting an epigenetic mechanism. This hypothesis is supported by changes in DNA methylation and microRNA expression patterns inflicted by early-life Pb exposure. Pb pollution in Mexico represents a significant problem because past and current mining activities, historical use of Pb as fuel additive and culturally rooted use of Pb in glazed ceramics, contribute to high levels of Pb pollution in Mexico. In this review we will discuss potential risks of AD development in Mexican populations chronically exposed to Pb in their childhood.

Similar content being viewed by others
References
Wortmann M (2012) Dementia: a global health priority-highlights from an ADI and World Health Organization report. Alzheimers Res Ther 4(5):40
Gutierrez-Robledo LM, Arrieta-Cruz I (2015) Dementia in Mexico: the need for a National Alzheimer s Plan. Gac Med Mex 151(5):667–673
Valencia-González D, Ramírez-Santos R, Acosta-Castillo GI (2017) Prevalence of dementia in a general hospital in Mexico City. Alzheimer’s Dement 13(, Supplement):P840
Iqbal K et al (2005) Tau pathology in Alzheimer disease and other tauopathies. Biochim Biophys Acta 1739(2–3):198–210
Hardy JA, Higgins GA (1992) Alzheimer’s disease: the amyloid cascade hypothesis. Science 256(5054):184–185
Balin BJ, Hudson AP (2014) Etiology and pathogenesis of late-onset Alzheimer’s disease. Curr Allergy Asthma Rep 14(3):417
Corder E et al (1993) Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science 261(5123):921–923
Jonsson T et al (2013) Variant of TREM2 associated with the risk of Alzheimer’s disease. N Engl J Med 368(2):107–116
Ulrich JD et al (2017) Elucidating the role of TREM2 in Alzheimer’s disease. Neuron 94(2):237–248
Serrano-Pozo A, Growdon JH (2019) Is Alzheimer’s disease risk modifiable? J Alzheimers Dis 67(3):795–819
Heusinkveld HJ et al (2016) Neurodegenerative and neurological disorders by small inhaled particles. Neurotoxicology 56:94–106
Chin-Chan M, Navarro-Yepes J, Quintanilla-Vega B (2015) Environmental pollutants as risk factors for neurodegenerative disorders: alzheimer and Parkinson diseases. Front Cell Neurosci 9:124
Hernandez Avila M et al (1991) Lead-glazed ceramics as major determinants of blood lead levels in Mexican women. Environ Health Perspect 94:117–120
Villalobos M et al (2009) Lead (II) detection and contamination routes in environmental sources, cookware and home-prepared foods from Zimatlan, Oaxaca. Mexico. Sci Total Environ 407(8):2836–2844
Tong S, McMichael AJ (1999) The magnitude, persistence and public health significance of cognitive effects of environmental lead exposure in childhood. J Environ Med 1(2):103–110
Caravanos J et al (2014) Blood lead levels in Mexico and pediatric burden of disease implications. Ann Glob Health 80(4):269–277
Bihaqi SW, Eid A, Zawia NH (2017) Lead exposure and tau hyperphosphorylation: an in vitro study. Neurotoxicology 62:218–223
Dobrakowski M et al (2017) Oxidative DNA damage and oxidative stress in lead-exposed workers. Hum Exp Toxicol 36(7):744–754
Gu H et al (2011) Lead exposure increases levels of beta-amyloid in the brain and CSF and inhibits LRP1 expression in APP transgenic mice. Neurosci Lett 490(1):16–20
Maloney B et al (2012) Applying epigenetics to Alzheimer’s disease via the latent early-life associated regulation (LEARn) model. Curr Alzheimer Res 9(5):589–599
Stoccoro A, Coppede F (2018) Role of epigenetics in Alzheimer’s disease pathogenesis. Neurodegener Dis Manag 8(3):181–193
Li YY et al (2012) Lead exposure in pheochromocytoma cells induces persistent changes in amyloid precursor protein gene methylation patterns. Environ Toxicol 27(8):495–502
Wu J et al (2008) Alzheimer’s disease (AD)-like pathology in aged monkeys after infantile exposure to environmental metal lead (Pb): evidence for a developmental origin and environmental link for AD. J Neurosci 28(1):3–9
Morris G et al (2019) Could Alzheimer’s disease originate in the periphery and if so how so? Mol Neurobiol 56(1):406–434
Raskin J et al (2015) Neurobiology of Alzheimer’s disease: integrated molecular, physiological, anatomical, biomarker, and cognitive dimensions. Curr Alzheimer Res 12(8):712–722
Collaborators GBDD (2019) Global, regional, and national burden of Alzheimer’s disease and other dementias, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 18(1):88–106
Snyder HM et al (2016) Alzheimer’s disease research in Ibero America. Alzheimer’s Dement 12(6):749–754
Talwar P et al (2016) Dissecting complex and multifactorial nature of Alzheimer’s disease pathogenesis: a clinical, genomic, and systems biology perspective. Mol Neurobiol 53(7):4833–4864
Dosunmu R et al (2007) Environmental and dietary risk factors in Alzheimer’s disease. Expert Rev Neurother 7(7):887–900
Chopra K, Misra S, Kuhad A (2011) Neurobiological aspects of Alzheimer’s disease. Expert Opin Ther Targets 15(5):535–555
Kumar K et al (2018) Recent advances in the neurobiology and neuropharmacology of Alzheimer’s disease. Biomed Pharmacother 98:297–307
Edwards FA (2019) A unifying hypothesis for Alzheimer’s disease: from plaques to neurodegeneration. Trends Neurosci 42(5):310–322
Selkoe DJ, Hardy J (2016) The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol Med 8(6):595–608
Gauthier S et al (2016) Why has therapy development for dementia failed in the last two decades? Alzheimers Dement 12(1):60–64
LaFerla FM, Green KN, Oddo S (2007) Intracellular amyloid-beta in Alzheimer’s disease. Nat Rev Neurosci 8(7):499–509
Haass C et al (2012) Trafficking and proteolytic processing of APP. Cold Spring Harb Perspect Med 2(5):a006270
Kametani F, Hasegawa M (2018) Reconsideration of amyloid hypothesis and tau hypothesis in Alzheimer’s disease. Front Neurosci 12:25
Murphy MP, LeVine H 3rd (2010) Alzheimer’s disease and the amyloid-beta peptide. J Alzheimer’s Dis JAD 19(1):311–323
Hardy J, Selkoe DJ (2002) The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science 297(5580):353–356
Timmers M et al (2019) Relevance of the interplay between amyloid and tau for cognitive impairment in early Alzheimer’s disease. Neurobiol Aging 79:131–141
LaFerla FM, Green KN (2012) Animal models of Alzheimer disease. Cold Spring Harb Perspect Med 2(11):a006320. https://doi.org/10.1101/cshperspect.a006320
Nalivaeva NN et al (2012) Are amyloid-degrading enzymes viable therapeutic targets in Alzheimer’s disease? J Neurochem 120(Suppl 1):167–185
Yasojima K et al (2001) Reduced neprilysin in high plaque areas of Alzheimer brain: a possible relationship to deficient degradation of beta-amyloid peptide. Neurosci Lett 297(2):97–100
Kanekiyo T, Bu G (2014) The low-density lipoprotein receptor-related protein 1 and amyloid-beta clearance in Alzheimer’s disease. Front Aging Neurosci 6:93
Pahnke J, Langer O, Krohn M (2014) Alzheimer’s and ABC transporters–new opportunities for diagnostics and treatment. Neurobiol Dis 72 Neurobiol Dis:54–60
Dolan PJ, Johnson GV (2010) The role of tau kinases in Alzheimer’s disease. Curr Opin Drug Discov Dev 13(5):595–603
Obulesu M, Venu R, Somashekhar R (2011) Tau Mediated neurodegeneration: an insight into Alzheimer’s disease pathology. Neurochem Res 36(8):1329–1335
Gonçalves RA et al (2019) The link between tau and insulin signaling: implications for Alzheimer’s disease and other tauopathies. Front Cell Neurosci 13:17. https://doi.org/10.3389/fncel.2019.00017
Baas PW, Qiang L (2019) Tau: it’s not what you think. Trends Cell Biol 29(6):452–461
Yu CC et al (2019) Epigenetic modulation on tau phosphorylation in Alzheimer’s disease. Neural Plast 2019:6856327
Satoh A, Iijima KM (2019) Roles of tau pathology in the locus coeruleus (LC) in age-associated pathophysiology and Alzheimer’s disease pathogenesis: potential strategies to protect the LC against aging. Brain Res 1702:17–28
Nam WH, Choi YP (2019) In vitro generation of tau aggregates conformationally distinct from parent tau seeds of Alzheimer’s brain. Prion 13(1):1–12
Gibbons GS et al (2019) Detection of Alzheimer’s disease (AD) specific tau pathology with conformation-selective anti-tau monoclonal antibody in co-morbid frontotemporal lobar degeneration-tau (FTLD-tau). Acta Neuropathol Commun 7(1):34
Spillantini MG, Goedert M (2013) Tau pathology and neurodegeneration. Lancet Neurol 12(6):609–622
Mandelkow E et al (2007) Structural principles of tau and the paired helical filaments of Alzheimer’s disease. Brain Pathol 17(1):83–90
Garcia ML, Cleveland DW (2001) Going new places using an old MAP: tau, microtubules and human neurodegenerative disease. Curr Opin Cell Biol 13(1):41–48
Goedert M, Spillantini MG (2000) Tau mutations in frontotemporal dementia FTDP-17 and their relevance for Alzheimer’s disease. Biochim Biophys Acta 1502(1):110–121
Egger G et al (2004) Epigenetics in human disease and prospects for epigenetic therapy. Nature 429(6990):457–463
Greenberg MVC, Bourc’his D (2019) The diverse roles of DNA methylation in mammalian development and disease. Nat Rev Mol Cell Biol 20(10):590–607
Jones PA (2012) Functions of DNA methylation: islands, start sites, gene bodies and beyond. Nat Rev Genet 13(7):484–492
Abdolmaleky HM et al (2004) Methylomics in psychiatry: modulation of gene–environment interactions may be through DNA methylation. Am J Med Genet Part B Neuropsychiatr Genet 127B(1):51–59
Friso S et al (2017) One-carbon metabolism and epigenetics. Mol Aspects Med 54:28–36
Bártová E et al (2008) Histone modifications and nuclear architecture: a review. J Histochem Cytochem 56(8):711–721
Li B, Carey M, Workman JL (2007) The role of chromatin during transcription. Cell 128(4):707–719
Cedar H, Bergman Y (2009) Linking DNA methylation and histone modification: patterns and paradigms. Nat Rev Genet 10(5):295–304
Ramakrishna S, Muddashetty RS (2019) Emerging role of microRNAs in dementia. J Mol Biol 431(9):1743–1762
Ha M, Kim VN (2014) Regulation of microRNA biogenesis. Nat Rev Mol Cell Biol 15(8):509–524
Quinlan S et al (2017) MicroRNAs in neurodegenerative diseases. Int Rev Cell Mol Biol 334:309–343
Goate A, Chartier-Harlin M-C (1991) Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer’s disease. Nature 349(6311):704
Scheuner D et al (1996) Secreted amyloid [beta]-protein similar to that in the senile plaques of Alzheimer’s disease is increased in vivo by the presenilin 1 and 2 and APP mutations linked to familial Alzheimer’s disease. Nat Med 2(8):864–870
Bertram L et al (2007) Systematic meta-analyses of Alzheimer disease genetic association studies: the AlzGene database. Nat Genet 39(1):17–23
Bird TD (2005) Genetic factors in Alzheimer’s disease. N Engl J Med 352(9):862–864
Gatz M et al (2006) Role of genes and environments for explaining alzheimer disease. Arch Gen Psychiatry 63(2):168–174
Bollati V et al (2009) Decline in genomic DNA methylation through aging in a cohort of elderly subjects. Mech Ageing Dev 130(4):234–239
Suarez NA, Macia A, Muotri AR (2018) LINE-1 retrotransposons in healthy and diseased human brain. Dev Neurobiol 78(5):434–455
Chouliaras L et al (2013) Consistent decrease in global DNA methylation and hydroxymethylation in the hippocampus of Alzheimer’s disease patients. Neurobiol Aging 34(9):2091–2099
Coppieters N et al (2014) Global changes in DNA methylation and hydroxymethylation in Alzheimer’s disease human brain. Neurobiol Aging 35(6):1334–1344
Bakulski KM et al (2012) Genome-wide DNA methylation differences between late-onset Alzheimer’s disease and cognitively normal controls in human frontal cortex. Journal of Alzheimer’s Disease 29(3):571–588
Mastroeni D et al (2010) Epigenetic changes in Alzheimer’s disease: decrements in DNA methylation. Neurobiol Aging 31(12):2025–2037
West RL, Lee JM, Maroun LE (1995) Hypomethylation of the amyloid precursor protein gene in the brain of an alzheimer’s disease patient. J Mol Neurosci 6(2):141–146
Rogaev EI et al (1994) The upstream promoter of the β-amyloid precursor protein gene (APP) shows differential patterns of methylation in human brain. Genomics 22(2):340–347
Tohgi H et al (1999) Reduction with age in methylcytosine in the promoter region −224-101 of the amyloid precursor protein gene in autopsy human cortex. Mol Brain Res 70(2):288–292
De Jager PL et al (2014) Alzheimery’s disease pathology is associated with early alterations in brain DNA methylation at ANK1, BIN1, RHBDF2 and other loci. Nat Neurosci 17(9):1156–1163
Wang S-C, Oelze B, Schumacher A (2008) Age-specific epigenetic drift in late-onset Alzheimer’s disease. PLoS One 3(7):e2698
Fuso A et al (2005) S-adenosylmethionine/homocysteine cycle alterations modify DNA methylation status with consequent deregulation of PS1 and BACE and beta-amyloid production. Mol Cell Neurosci 28(1):195–204
Gupta S et al (2010) Histone methylation regulates memory formation. J Neurosci 30(10):3589–3599
Kular RK et al (2009) Neuronal differentiation is regulated by leucine-rich acidic nuclear protein (LANP), a member of the inhibitor of histone acetyltransferase complex. J Biol Chem 284(12):7783–7792
Lu X et al (2014) Histone acetyltransferase p300 mediates histone acetylation of PS1 and BACE1 in a cellular model of Alzheimer’s disease. PLoS One 9(7):e103067
Lu X et al (2015) Histone acetylation modifiers in the pathogenesis of Alzheimer’s disease. Front Cell Neurosc 9:226. https://doi.org/10.3389/fncel.2015.00226
Narayan PJ et al (2015) Increased acetyl and total histone levels in post-mortem Alzheimer’s disease brain. Neurobiol Dis 74:281–294
Williams AH et al (2009) MicroRNA-206 delays ALS progression and promotes regeneration of neuromuscular synapses in mice. Science 326(5959):1549
Khudayberdiev S, Fiore R, Schratt G (2009) MicroRNA as modulators of neuronal responses. Commun Integr Biol 2(5):411–413
Long JM, Ray B, Lahiri DK (2014) MicroRNA-339-5p down-regulates protein expression of beta-site amyloid precursor protein-cleaving enzyme 1 (BACE1) in human primary brain cultures and is reduced in brain tissue specimens of Alzheimer disease subjects. J Biol Chem 289(8):5184–5198
Long JM, Lahiri DK (2011) MicroRNA-101 downregulates Alzheimer’s amyloid-beta precursor protein levels in human cell cultures and is differentially expressed. Biochem Biophys Res Commun 404(4):889–895
Long JM, Ray B, Lahiri DK (2012) MicroRNA-153 physiologically inhibits expression of amyloid-beta precursor protein in cultured human fetal brain cells and is dysregulated in a subset of Alzheimer disease patients. J Biol Chem 287(37):31298–31310
Hébert SS et al (2008) Loss of microRNA cluster miR-29a/b-1 in sporadic Alzheimer’s disease correlates with increased BACE1/β-secretase expression. Proc Natl Acad Sci 105(17):6415–6420
Yegambaram M et al (2015) Role of environmental contaminants in the etiology of Alzheimer’s disease: a review. Curr Alzheimer Res 12(2):116–146
Guan C et al (2017) Characterization of plasma metal profiles in Alzheimer’s disease using multivariate statistical analysis. PLoS One 12(7):e0178271
Horton CJ, Weng H-Y, Wells EM (2019) Association between blood lead level and subsequent Alzheimer’s disease mortality. Environ Epidemiol 3(3):e045
Park JH et al (2014) Serum trace metal levels in Alzheimer’s disease and normal control groups. Am J Alzheimers Dis Other Demen 29(1):76–83
Brown EE et al (2019) Lead (Pb) in Alzheimer’s dementia: a systematic review of human case-control studies. Curr Alzheimer Res 16(4):353–361
Stewart WF et al (2006) Past adult lead exposure is linked to neurodegeneration measured by brain MRI. Neurology 66(10):1476–1484
Stewart WF, Schwartz BS (2007) Effects of lead on the adult brain: a 15-year exploration. Am J Ind Med 50(10):729–739
Prada D et al (2016) APOE epsilon4 allele modifies the association of lead exposure with age-related cognitive decline in older individuals. Environ Res 151:101–105
Rosin A (2009) The long-term consequences of exposure to lead. Isr Med Assoc J 11(11):689–694
Zawia NH, Lahiri DK, Cardozo-Pelaez F (2009) Epigenetics, oxidative stress, and Alzheimer disease. Free Radic Biol Med 46(9):1241–1249
Basha MR et al (2005) The fetal basis of amyloidogenesis: exposure to lead and latent overexpression of amyloid precursor protein and beta-amyloid in the aging brain. J Neurosci 25(4):823–829
Bihaqi SW et al (2014) Infantile exposure to lead and late-age cognitive decline: relevance to AD. Alzheimers Dement 10(2):187–195
Engstrom AK et al (2017) Gene-environment interaction between lead and apolipoprotein E4 causes cognitive behavior deficits in mice. Mol Neurodegener 12(1):14
Wallin C et al (2017) Alzheimer’s disease and cigarette smoke components: effects of nicotine, PAHs, and Cd(II), Cr(III), Pb(II), Pb(IV) ions on amyloid-beta peptide aggregation. Sci Rep 7(1):14423
Meleleo D et al (2019) Concentration-dependent effects of mercury and lead on Abeta42: possible implications for Alzheimer’s disease. Eur Biophys J 48(2):173–187
Bihaqi SW et al (2014) Infantile postnatal exposure to lead (Pb) enhances tau expression in the cerebral cortex of aged mice: relevance to AD. Neurotoxicology 44:114–120
Bihaqi SW, Zawia NH (2013) Enhanced taupathy and AD-like pathology in aged primate brains decades after infantile exposure to lead (Pb). Neurotoxicology 39:95–101
Liu F et al (2014) Effects of lead exposure on the expression of amyloid beta and phosphorylated tau proteins in the C57BL/6 mouse hippocampus at different life stages. J Trace Elem Med Biol 28(2):227–232
Li N et al (2016) The effects of early life lead exposure on the expression of glycogen synthase kinase-3beta and insulin-like growth factor 1 receptor in the hippocampus of mouse pups. Biol Trace Elem Res 169(1):114–120
Gassowska M et al (2016) Perinatal exposure to lead (Pb) promotes Tau phosphorylation in the rat brain in a GSK-3beta and CDK5 dependent manner: relevance to neurological disorders. Toxicology 347–349:17–28
Li N et al (2014) The effects of early life Pb exposure on the expression of IL1-beta, TNF-alpha and Abeta in cerebral cortex of mouse pups. J Trace Elem Med Biol 28(1):100–104
Huang H et al (2011) In vitro Pb exposure disturbs the balance between Abeta production and elimination: the role of AbetaPP and neprilysin. Neurotoxicology 32(3):300–306
Chin-Chan M et al (2015) Mercury reduces the enzymatic activity of neprilysin in differentiated SH-SY5Y Cells. Toxicol Sci 145(1):128–137
Deng Z et al (2015) Effects of selenium on lead-induced alterations in Abeta production and Bcl-2 family proteins. Environ Toxicol Pharmacol 39(1):221–228
Ashok A et al (2015) Exposure to As-, Cd-, and Pb-mixture induces Abeta, amyloidogenic APP processing and cognitive impairments via oxidative stress-dependent neuroinflammation in young rats. Toxicol Sci 143(1):64–80
Behl M, Zhang Y, Zheng W (2009) Involvement of insulin-degrading enzyme in the clearance of beta-amyloid at the blood-CSF barrier: consequences of lead exposure. Cerebrospinal Fluid Res 6:11
Li N et al (2016) Decreased IDE and IGF2 expression but increased Abeta40 in the cerebral cortex of mouse pups by early life lead exposure. Brain Res Bull 121:84–90
Behl M et al (2010) Lead-induced accumulation of beta-amyloid in the choroid plexus: role of low density lipoprotein receptor protein-1 and protein kinase C. Neurotoxicology 31(5):524–532
Barker DJ (2004) The developmental origins of chronic adult disease. Acta Paediatr Suppl 93(446):26–33
Vaiserman A (2015) Epidemiologic evidence for association between adverse environmental exposures in early life and epigenetic variation: a potential link to disease susceptibility? Clin Epigenet 7(1):96
Bommarito PA, Martin E, Fry RC (2017) Effects of prenatal exposure to endocrine disruptors and toxic metals on the fetal epigenome. Epigenomics 9(3):333–350
Sen A et al (2015) Early life lead exposure causes gender-specific changes in the DNA methylation profile of DNA extracted from dried blood spots. Epigenomics 7(3):379–393
Toraño EG et al (2016) The impact of external factors on the epigenome: in utero and over lifetime. Biomed Res Int 2016:2568635. https://doi.org/10.1155/2016/2568635
An J et al (2014) The changes of miRNA expression in rat hippocampus following chronic lead exposure. Toxicol Lett 229(1):158–166
Luo M et al (2014) Epigenetic histone modification regulates developmental lead exposure induced hyperactivity in rats. Toxicol Lett 225(1):78–85
Wu J, Basha MR, Zawia NH (2008) The environment, epigenetics and amyloidogenesis. J Mol Neurosci 34(1):1–7
Mazumdar M et al (2012) Prenatal lead levels, plasma amyloid beta levels, and gene expression in young adulthood. Environ Health Perspect 120(5):702–707
Lee J, Freeman JL (2014) Zebrafish as a model for investigating developmental lead (Pb) neurotoxicity as a risk factor in adult neurodegenerative disease: a mini-review. Neurotoxicology 43:57–64
Lee J, Freeman JL (2016) Embryonic exposure to 10 μg/L lead results in female-specific expression changes in genes associated with nervous system development and function and Alzheimer’s disease in aged adult zebrafish brain. Metallomics 8(6):589–596
Bihaqi SW et al (2011) Infant exposure to lead (Pb) and epigenetic modifications in the aging primate brain: implications for Alzheimer’s disease. J Alzheimers Dis 27(4):819–833
Bihaqi SW (2019) Early life exposure to lead (Pb) and changes in DNA methylation: relevance to Alzheimer’s disease. Rev Environ Health 34(2):187–195
Bihaqi SW, Zawia NH (2012) Alzheimer’s disease biomarkers and epigenetic intermediates following exposure to Pb in vitro. Curr Alzheimer Res 9(5):555–562
Masoud AM et al (2016) Early-life exposure to lead (Pb) alters the expression of microRNA that target proteins associated with Alzheimer’s disease. J Alzheimers Dis 51(4):1257–1264
Eid A et al (2016) Developmental lead exposure and lifespan alterations in epigenetic regulators and their correspondence to biomarkers of Alzheimer’s disease. Alzheimers Dement (Amst) 2:123–131
Flores J, Albert LA (2004) Environmental lead in Mexico, 1990-2002. Rev Environ Contam Toxicol 181:37–109
Gonzalez de Mejia E, Craigmill AL (1996) Transfer of lead from lead-glazed ceramics to food. Arch Environ Contam Toxicol 31(4):581–584
Hernández-Serrato MI et al (2003) Factors associated with lead exposure in Oaxaca, Mexico. J Eposure Sci Environ Epidemiol 13(5):341–347
Flores-Ramírez R et al (2012)., Exposición infantil al plomo en sitios contaminados. Salud Pública de México 54(4):383–392
Armienta MA et al (2003) Geochemistry of metals from mine tailings in Taxco, Mexico. Bull Environ Contam Toxicol 71(2):387–393
Moreno ME et al (2010) Biomonitoring of metal in children living in a mine tailings zone in Southern Mexico: a pilot study. Int J Hyg Environ Health 213(4):252–258
Benin AL et al (1999) High concentrations of heavy metals in neighborhoods near ore smelters in northern Mexico. Environ Health Perspect 107(4):279–284
Pantic I et al (2018) Children’s blood lead concentrations from 1988 to 2015 in Mexico City: the contribution of lead in air and traditional lead-glazed ceramics. Int J Environ Res Public Health. https://doi.org/10.3390/ijerph15102153
Tong S, Schirnding YEV, Prapamontol T (2000) Prapamontol, environmental lead exposure: a public health problem of global dimensions. Bull World Health Org 78(9):1068–1077
SSA, Secretaria de Salud Norma Oficial Mexicana NOM-199-SSAI-2000, Salud Ambiental. Niveles de plomo en Sangre y acciones como criterios para proteger la salud de la poblaci[on expuesta no ocupacionalmente., In: Mexico. Diario Oficial de la Federacion 2002
CDC (2017) CDC’s Childhood Lead Poisoning Prevention Program. 2017. https://www.cdc.gov/nceh/lead/acclpp/blood_lead_levels.htm. Accessed 30 July 2019
EPA (2017) Basic Information about Lead in Drinking Water. United States Environmental Protection Agency, 2017
Romieu I et al (1995) Environmental urban lead exposure and blood lead levels in children of Mexico City. Environ Health Perspect 103(11):1036–1040
Jiménez C et al (2006) Factores de exposición ambiental y concentraciones de plomo en sangre en niños de la Ciudad de México. Salud Pública de México 35(6):599–606
Téllez-Rojo MM et al (2006) Longitudinal associations between blood lead concentrations lower than 10 μg/dl and neurobehavioral development in environmentally exposed children in Mexico City. Pediatrics 118(2):e323–e330
Terrazas-Meraz MA et al (2015) Use of lead-glazed ceramic as a source of exposure in children of marginalized indigenous zones of Oaxaca, Mexico. Salud Publ Mex 57(3):260–264
Tellez-Rojo MM et al (2017) Lead poisoning and marginalization in newborns of Morelos, Mexico. Salud Publ Mex 59(3):218–226
Soto-Jimenez MF, Flegal AR (2011) Childhood lead poisoning from the smelter in Torreon, Mexico. Environ Res 111(4):590–596
Garcia-Vargas GG et al (2014) Spatial clustering of toxic trace elements in adolescents around the Torreon, Mexico lead-zinc smelter. J Expo Sci Environ Epidemiol 24(6):634–642
Gamino-Gutierrez SP et al (2013) Arsenic and lead contamination in urban soils of Villa de la Paz (Mexico) affected by historical mine wastes and its effect on children’s health studied by micronucleated exfoliated cells assay. Environ Geochem Health 35(1):37–51
Castro Gonzalez NP et al (2017) Assessment risk to children’s health due to consumption of cow’s milk in polluted areas in Puebla and Tlaxcala, Mexico. Food Addit Contam Part B Surveill 10(3):200–207
Perng W et al (2019) Early Life Exposure in Mexico to ENvironmental Toxicants (ELEMENT) Project. BMJ Open 9(8):e030427
Tamayo-Ortiz M, Navia-Antezana J (2018) Reduced lead exposure following a sensitization program in rural family homes producing traditional Mexican ceramics. Ann Glob Health 84(2):285–291
Garcia Vargas GG et al (2001) Lead exposure in children living in a smelter community in region Lagunera, Mexico. J Toxicol Environ Health A 62(6):417–429
Acosta-Saavedra LC et al (2011) Environmental exposure to lead and mercury in Mexican children: a real health problem. Toxicol Mech Methods 21(9):656–666
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chin-Chan, M., Cobos-Puc, L., Alvarado-Cruz, I. et al. Early-life Pb exposure as a potential risk factor for Alzheimer’s disease: are there hazards for the Mexican population?. J Biol Inorg Chem 24, 1285–1303 (2019). https://doi.org/10.1007/s00775-019-01739-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00775-019-01739-1