Abstract
Background
Antenatal corticosteroids (ACSs) are recommended to all women at risk for preterm delivery; currently, there is controversy about the subsequent long-term neurocognitive sequelae. This systematic review summarizes the long-term neurodevelopmental outcomes after ACS therapy in animal models.
Methods
An electronic search strategy incorporating MeSH and keywords was performed using all known literature databases and in accordance with PRISMA guidance (PROSPERO CRD42019119663).
Results
Of the 669 studies identified, eventually 64 were included. The majority of studies utilized dexamethasone at relative high dosages and primarily involved rodents. There was a high risk of bias, mostly due to lack of randomization, allocation concealment, and blinding. The main outcomes reported on was neuropathological, particularly glucocorticoid receptor expression and neuron densities, and neurobehavior. Overall there was an upregulation of glucocorticoid receptors with lower neuron densities and a dysregulation of the dopaminergic and serotonergic systems. This coincided with various adverse neurobehavioral outcomes.
Conclusions
In animal models, ACSs consistently lead to deleterious long-term neurocognitive effects. This may be due to the specific agents, i.e., dexamethasone, or the repetitive/higher total dosing used. ACS administration varied significantly between studies and there was a high risk of bias. Future research should be standardized in well-characterized models.
Similar content being viewed by others
Background
Glucocorticoids (GCs) are essential in the biological processes required for the transition from intrauterine to extrauterine life. The overall action of endogenous GCs is to trigger organ maturation, thereby enabling the lungs, liver, gastrointestinal tract, thyroid, adrenals, and kidneys to function and sustain life outside the uterine environment.1 GCs are also crucial for normal brain maturation, as they initiate terminal maturation, remodel axons and dendrites, and affect cell survival.2 Both suppressed and elevated GC levels can impair brain development and functioning.3
Since 1994, antenatal corticosteroids (ACSs) have been recommended to all women at risk for delivery between 24 and 34 weeks of gestation,4 as ACSs are effective not only in reducing perinatal morbidity, i.e., respiratory distress syndrome, intraventricular hemorrhage, necrotizing enterocolitis, and sepsis, but also the mortality that is associated with prematurity.5 Although the beneficial short-term outcomes of ACS therapy were evident from an early-stage, longer-term outcomes, including neurodevelopment, have been less extensively studied. A systematic review of maternal ACS administration in pregnancy reported improved neurodevelopmental outcomes in these children. However, this systematic review consisted mostly of nonrandomized studies and reported on crude neurodevelopmental outcomes.6 Therefore, although ACS therapy appears safe and effective, current clinical data cannot define the precise effect of ACS therapy on future neurodevelopment. Long-term effects of ACS therapy have recently been described in a longitudinal study suggesting that ACS therapy yields persistent changes in hypothalamic–pituitary–adrenal (HPA) axis reactivity into late adolescence and may confer increased vulnerability for developing stress-related disorders.7
In view of the unclear long-term outcomes in clinical studies and the widespread use of ACSs, it is reasonable to reflect on animal studies to guide future research. Despite a number of preclinical studies investigating the neurocognitive effect of ACSs, the majority have reported only on direct or short-term effects.8,9 In addition, the effects being investigated are not standardized or consistent between studies. To date, there has been no systematic review summarizing the long-term neurodevelopmental outcomes after ACS therapy in preclinical models.
Methods
Protocol and registration
This systematic review was performed in accordance with the Preferred Reporting Items for Systematic reviews and Meta-analyses guidance.10 The protocol was registered with the International Prospective Register of Systematic Reviews (PROSPERO) (CRD42019119663).
Literature search strategy
A literature search was conducted in PubMed, MEDLINE, EMBASE, Scopus, Web of Science, and the Cochrane Library. The electronic search strategy included both Medical Subject Headings (MeSH) and keywords (Supplementary Information 1). Reference lists and topic-related reviews were checked manually to identify further relevant papers. Zotero 5.0 (George Mason University, VA, USA) was used to coordinate study screening and data collection.
Inclusion and exclusion criteria
All studies reporting on the use of ACSs in animals were considered eligible. No date or language restrictions were applied. Systematic reviews, narrative review articles, and editorials were excluded. Studies were excluded if corticosteroids were administered postnatally and if no long-term or neurological outcomes were reported in the offspring. For the purposes of this study, a postnatal age of ≥7 days was considered as long term. This empiric cut-off was introduced since no interspecies long-term definition exist, and we wanted to exclude studies that reported on the acute effects of ACS. Neurological outcomes were defined as any neuropathological, neurobehavioral, or neuroimaging (i.e., computed tomography (CT) and magnetic resonance imaging (MRI)) results.
Study selection
J.L.v.d.M. and A.S. independently screened titles and abstracts and thereafter performed a full-text review of all studies. Disagreements were resolved by consensus. A low threshold for full-text retrieval and review was used.
Data extraction
J.L.v.d.M. and A.S. independently extracted data and entered this into a standardized Excel (Microsoft Corp, Seattle, Washington, USA) form (Supplementary Information 2). Disagreements were resolved by consensus. Information noted included study design, animal species, number of animals, and gestation for that model. Treatment data recorded included type of corticosteroid, route of administration, number of doses, and gestational age of treatment. Treatment regimens were grouped into those administering a “single course” of corticosteroids, i.e., a single or two doses given within 48 h of each other and those administering “multiple courses” of corticosteroids, i.e., repeated doses given over >2 days. The dose of corticosteroids given was noted and converted into mg/kg based on information provided in the study, if not already given as such. This was then multiplied by the number of doses given in order to give a total administered dose in mg/kg. The clinical recommended intramuscularly dose for betamethasone (BM) is 24 mg in two divided doses 24 hours apart and dexamethasone (DM) 24 mg in four divided doses 12 hours apart.11 Hence the calculated average human total dose is 0.4 mg/kg (for the average female weight of 60-80 kg) and based upon body surface area an equivalent dose in animals also comes to 0.4 mg/kg.12 We therefore grouped the total study dosing regimens into one of the following: < 0.2 mg/kg, 0.2–0.4 mg/kg (clinical equivalent dosing), 0.41–1.0 mg/kg, and >1.0 mg/kg. Outcome data recorded included age of animals at assessment, neuropathology parameters, neurobehavioral and neuroimaging outcomes, as well as the overall effect of ACS in that study.
Risk of bias
Risk of bias was independently assessed by J.L.v.d.M. and A.S. using the Systematic Review Centre for Laboratory Animal Experimentation’s tool for animal interventional studies.13 Study quality was noted on a standardized Excel form. Disagreements were resolved by consensus.
Data synthesis and statistical methods
Meta-analysis and comparative statistics were not planned as it was anticipated that the data would be difficult to collate or compare. Therefore, heterogeneity between studies was not calculated and narrative results and descriptive statistics were produced.
Results
Study selection
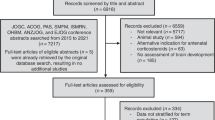
The electronic search identified 575 studies published until October 2018 (Fig. 1); hand-searching of reference lists identified a further 94 studies. Following removal of duplicate studies (273), 396 studies were screened by title and abstract and a further 286 were excluded as irrelevant. Full-text review of the remaining 110 studies was conducted, and 46 were excluded. The main reasons for study exclusion at any stage were: no ACSs given (56.6%, 188/332); no long-term outcomes (16.0%, 53/332); reviews and editorials (14.8%, 49/332); and no neuropathological, neurobehavioral, or neuroimaging outcomes (9.6%, 32/332). After exclusions, 64 studies were included for systematic review.
Flow diagram of the study selection adapted from PRISMA 2010.10
Study characteristics
Characteristics of the included studies are shown in Table 1. The majority of studies were in the rat (70.3%, 45/64); other animal models included the mouse (14.1%, 9/64), non-human primates (NHPs; 6.25%, 4/64) the sheep (4.7%, 3/64), and the guinea pig (4.7%, 3/64). Most studies (93.8%, 60/64) compared corticosteroids to a non-active control (e.g., saline), although 4 studies (6.3%) compared corticosteroids to no treatment. All included studies evaluated animals born at term gestations.
Risk of bias
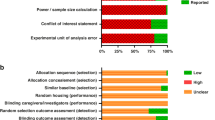
Risk of bias of the included studies is shown in Fig. 2. Most studies had a high risk of bias due to the lack of random sequence generation (65.7%, 42/64), allocation concealment performed (75%, 48/64), and blinding of caregivers (10.9%, 7/64) or assessors (29.7%, 19/64).
Risk of bias assessment using SYRCLE’s risk of bias tool for animal studies.13
ACS treatment
Details of ACS treatment is shown in Table 2. Overall, DM was the most commonly studied corticosteroid (81.3%, 52/64); BM was used in 17.2% of studies (11/64) and both corticosteroids were used in one mouse study. Two thirds of studies administered multiple courses of corticosteroids (67.2%, 43/64) with the total administered dose varying from 0.1 to 70 mg/kg. Eighteen studies (28.1%) administered a total dose of corticosteroids which was equivalent to that used in humans (0.2–0.4 mg/kg) while the majority of studies (76.6%, 49/64) administered a total dose >0.4 mg/kg. In two studies (3.1%, 2/64), the effects of a brief low-dosage ACS exposure was also explored.
Growth—body weight
There was no routine reporting on the general health of the animals at the time of assessment or harvesting, though 50.0% (32/64) of the studies did report on the body weight at the time of the last assessment. Only one study reported an increased weight at the time of harvesting, a study of NHP using a multiple-day high DM dose.14 While 16 reported a decrease in body weight in those exposed to ACSs, herein DM was used in 13/1615,16,17,18,19,20,21,22,23,24,25,26,27 and BM only in 3/16.28,29,30 Furthermore, only 10.9% (7/64) reported on the brain weight or volume at the time of harvesting wherein 57.1% (4/7) reported a decrease of brain weight after the exposure of ACS.17,30,31,32
Outcome assessment
Neuropathology was the commonest outcome reported, either alone (28.1%, 18/64) or in combination with neurobehavioral assessment (42.2%, 27/64) or neuroimaging (3.1%, 2/64). Neurobehavioral assessment alone was assessed in 25.0% of studies (16/64), and one study (1.6%) assessed all three outcomes (Fig. 3). The average age at final assessment was 157 days (range 10–1800 days).
Neuropathological assessments performed
In the 48 studies that reported on a neuropathological outcomes, DM was most commonly used (79.2%, 38/48). In addition, in a third (35.4%, 17/48) of the neuropathological outcome studies, ACS was used at a clinical equivalent dose while the majority of studies (60.4%, 29/48) used an accumulative dose >0.4 mg/kg. The commonest neuropathological outcomes reported was glucocorticoid receptor (GR) quantification (29.2%, 14/48), neuron density (16.7%, 8/48), or a form of dendritic assessment (16.7%, 8/48). The complete breakdown of reported neuropathological outcomes are displayed in Table 3.
Neurobehavioral assessments performed
Neurobehavioral assessments were reported in 44 studies in only 3 of the species. As noted above, most studies reported on the effects of DM and used almost exclusively rat and mice species. Neurobehavioral outcomes assessed are summarized in Table 4.
Neuroimaging assessments performed
In one study, CT imaging was used to quantify total brain volumes in rats.33 Herein antenatal DM did not lead to any difference in brain volumes at 3 months of ages. A further two studies utilized MRI to quantify the hippocampal volumes and T2-signal intensities in the rat34 and NHP.35 In both of these studies, antenatal DM at a dose of 0.80 and 10 mg/kg, respectively, resulted in lower hippocampal volumes.
Discussion
To date, there has been no systematic review summarizing the long-term neurodevelopmental outcomes after ACS therapy in preclinical models. From this review, intrauterine exposure to synthetic GCs led consistently to deleterious long-term neurocognitive effects. These outcomes may be due to the specific agents, i.e., DM, or the repetitive or higher total dosing used. ACS administration varied significantly between studies and most studies suffered from a high risk of bias. Neuropathological outcomes were most commonly reported, specifically the expression of GR, while reduced neurobehavioral functioning was reported in mainly rodent species.
Synthetic GCs are agonists of the GR and predominantly act via genomic effects mediated by the GR, a nuclear transcription factor. Because of its marked GR expression, the fetal lung is one of the primary targets of synthetic GCs administered to expedite fetal development. The effects of ACSs on the fetal and neonatal lung have been reviewed elsewhere,5 but their impact on other organ systems with high GR expression including the brain and kidney have mostly been assessed in short-term outcomes.9 In this review, the commonest long-term neuropathological outcome reported on was the expression of GR in mostly the hippocampus and hypothalamus. Herein, both BM and DM in small and large animal models mainly induced an upregulation of GRs,21,26,36,37,38,39 although four of these studies used multiple DM dosing. However, in two studies a downregulation of GR was noted although in these an oral multiple day DM dosing was used.16,40 The ultimate effect of this dysregulation on the GR can have a significant inhibitory downstream effect on the developing fetal brain and HPA axis, leading to profound programming influences on the nervous system and henceforth an increase in the risk for emotional and cognitive impairments.41 The possible mechanisms involved are depicted in Fig. 4.
The human placenta expresses the genes for proopiomelanocortin and the major stress hormone, corticotropin-releasing hormone (CRH). As pregnancy progresses, these stress hormones including maternal cortisol, increase dramatically. Because of the positive feedback between GCs and placental CRH, the effects of excess endogenous or synthetic GCs may be amplified with potentially negative consequences for the developing fetus. The consequences of prenatal treatment with BM or DM may be more profound as they cross the placenta more easily because they are not readily metabolized by the placental enzyme, 11β-hydroxysteroid dehydrogenase type 2 (11β-HSD2), that protects the fetus from maternal cortisol.81,82 These synthetic GCs can gain direct access to glucocorticoid receptors without significant reduction in their circulating or tissue levels due to local oxidation. These endocrine changes are important for fetal maturation, but if the levels are altered (e.g., ACS exposure), they influence (program) the fetal nervous system, especially the meso-cortico-limbic system with long-term consequences.
GCs are critical for normal brain development, exerting direct effects on neuronal growth, cell to cell interactions, and neuronal reorganization.42 The mechanisms regulating the maturational effects of GCs on various fetal organs are complex. However, exposure of the developing brain to inappropriate levels of GCs at critical developmental time windows can modify both the structure and function of neuronal cells. The majority of studies used multiple or repetitive doses over multiple days, consequently in most studies there were a high total dose exposure of ≥0.4 mg/kg. Previously in small animal studies, ACS was associated with delayed growth of the whole body and brain, as well as altered behavior studies at birth.43,44 From this review, there was an inconsistent long-term impact on brain and/or body weight and size. In those studies that used a total dose of ≤0.4 mg/kg, ACS exposure was not associated with a reduction of long-term body or brain weight.45,46,47,48,49,50 However, in one study a single course of BM exposure led to a significant reduction in both body and brain weights.30 In sheep, fetal exposure to repeated doses of maternal BM results in significant reductions in fetal brain weight that persist until 3 years of age.51
Gross changes in brain growth are the result of specific alterations in neuronal development and cell death. It has previously been noted that the cellular proliferation in the brain of neonatal rats is acutely decreased by BM treatments and reductions in brain weight persist until at least 3 weeks of postnatal age.47 As with prenatal stress exposure, ACS can also influence fetal brain development by changing neuronal migration, synaptic plasticity, and neurotransmitter activity.52 In this review, some studies observed altered neuronal states that lead to persistent lower neuron densities especially in the hippocampus26,35 with ongoing amplified apoptosis53 and decreased proliferation48,49,54,55 being reported. Furthermore, the protective negative feedback loop of the HPA axis is mediated by cortisol binding to receptors in especially the hypothalamus, hippocampus, and prefrontal cortex. It was foreseeable that most studies in this review noted alterations in these specific regions.
Inhibiting or turning off the HPA response axis can lead to a direct effect on the dopaminergic and serotonergic systems. Studies noted that ACS exposure was associated with less dopaminergic cells in multiple brain regions including the amygdala and hypothalamus45,55,56,57,58,59 that also has an effect on the central norepinephrine and peripheral activation of the sympathetic nervous system.60 The meso-cortico-limbic system, mediated by dopamine release especially from the nucleus accumbens and ventral tegmental area, encodes the rewarding and reinforcing properties of natural reward behavior.61 Evidently any dysfunction of this system could lead to multiple neuropsychiatric conditions.
Moreover, the reprogramming of the HPA axis by ACS is also associated with lower neuron expression in the serotonergic system especially in the hypothalamus, hippocampus, and frontal cortex.45,58,62 The serotoninergic system, a neurotransmitter system implicated in stress regulation and etiology of affective disorders is therefore another target for ACS exposure.59 This review confirms the alterations in serotonin receptors (5-HT1A and 5-HT2A) and transporters secondary to hippocampal and hypothalamus GR programming.
In addition, ACS can also lead to altered glial astrocyte function. Astrocytes assume multiple roles in maintaining an optimally suited milieu for neuronal function from the production of trophic factors, regulation of neurotransmitters and ion concentrations to the removal of toxins and debris from the cerebrospinal fluid.63 Impairments in these and other functions, as well as physiological reactions of astrocytes to injury, can trigger or exacerbate neuronal dysfunction. In this review, multiple studies noted the long-term dysregulation of neuroglia, especially astrocytes.53,64,65
So far, it is clear that the developing brain, with the meso-cortico-limbic system as a focus point, is particularly sensitive to exogenous GCs. The hippocampus, which plays a central role in this system, has a myriad of complex functions within the brain. These include cognition, behavior, memory, coordination of the autonomic activity, and regulation of a number of endocrine systems.66,67 Given this wide spectrum of regulatory roles, it is apparent that it will have a profound impact in postnatal and adult life. In rats, prenatal DM exposure resulted in more anxiety-like behavior,22,31,45 sex-specific alterations in motor activity and sexual behavior,15,59 and impaired spatial memory.17,20 While in mice, maternal administration of ACS resulted in delayed development and impaired motoric function in the offspring.68 Even a single course of ACS resulted in affecting anxiety, memory, and socialization behaviors.32,65 In NHP, there was reduced sociability and increased motivation reward behavior69 with dramatic differences in the hippocampal structure and developmental as quantified by MRI.35
These results indicate that both DM and BM interfere with the developing brain but it remains unclear from clinical trials whether one corticosteroid (or one particular regimen) has advantages over another.70 Both cross the placenta in their active form, and both have similar biologic activity with neither acting as a mineralocorticoid and both having weak immunosuppressive short-term effects.68 BM and DM differ only in the configuration of a single methyl group with subsequent different pharmacokinetics; BM has a larger volume of distribution and decreased clearance and thus a longer half-life.71 In addition, the commonly available DM preparation contains a sodium metabisulfite preservative, and sulfite is neurotoxic. However, prenatal exposure to sulfite is likely to be low (because it is administered to the mother and may not reach the fetus at the same dose).72 From this review, both BM and DM resulted in long-term sequelae, with no clear benefit of one over another. As previously stated, most investigations used DM and mostly multiple dosing or courses, but even low dose or single courses of BM28,30,31,47,50,73 and DM32,48,49,55 resulted in clear neuropathological and neurobehavioral deficits.
This systematic review and the assimilated research have limitations mostly due to the administration regimes chosen, the lack of stringent methodological approach, and non-standardized reporting. The dosing regimens are not clearly “clinical equivalent” using mostly multiple dosages over multiple days. The true fetal exposure is not quantified and therefore the differences that each species’ metabolism bring is not addressed. Most of the studies investigate DM while BM is more in clinical use. There was also a high bias risk due the lack of randomization, allocational and treatment concealment, and ultimately selective/incomplete outcome reporting limit the interpretation of these results. Pseudoreplication is another critical methodological issue in animal behavioral research. Although many articles controlled for baseline characteristics, at least in some part, very few clearly stated how litter allocation was managed in their study. As with all translational research there is inherent risk that the risk or benefit can be overestimated due to publication bias. Ultimately, there is always the problem of species-specific factors that influence the translational value of the relevant research.
To grasp the effect of ACS on the neuroendocrine maturation, the timing of maturation of the HPA axis relative to birth needs to be clearly comprehended. In animals that give birth to mature young (sheep, guinea pigs, and primates), maximal brain growth and a large proportion of neuroendocrine maturation (including corticosteroid receptor development) takes place in utero.74,75 In contrast, in species that give birth to immature young (rats, rabbits, and mice), much neuroendocrine development occurs in the postnatal period.76 Therefore, maternal GC treatment in late gestation will impact on different stages of brain and HPA development depending on the species studied. Another important consideration when extrapolating among different studies and species is that of receptor sensitivity. Mice and rats are corticosensitive (high receptor affinity for GCs) compared with other species, such as guinea pigs and primates, which are considered corticoresistant.77
Conclusion
In conclusion, many animal models have been used to highlight the efficacy and potential adverse effects of ACS. In this review, a general pattern is observed of consistent neurocognitive sequelae that ultimately lead to modulated fetal programming, the so-called Developmental Origins of Health and Disease hypothesis. The mechanistic view of an intrauterine factor mediating brain growth and neurocognitive development at a vulnerable time in gestation while subsequently resulting in permanent alterations is one that has been included in many neurocognitive and psychiatric conditions. Current research pertaining to the neurocognitive effects of ACS consisted mostly of DM using repetitive and high dosages in rodent species. There is a new focus on ACS since the role and indication for ACS has recently rapidly expanded to include rescue and repeated dosages and late preterm birth.78,79,80
Preclinical research could help in defining the efficacy and long-term outcomes in future ACS research, but models needs to be standardized to help address the barriers in translational neuroscience research. Principles that could be followed are:
-
1.
Adequate and appropriate dosages that could include dose–response curves;
-
2.
Define the time and gestational age window of exposure in a well-characterized model;
-
3.
Blinded, physiologically controlled studies; and
-
4.
Histological and functional outcomes assessed acutely and long term.
Furthermore imaging, especially MRI, could help characterize the insults even better, especially in longitudinal models where the long-term impact needs to be defined. So far, this has been underutilized in this area.
References
Fowden, A. L., Li, J. & Forhead, A. J. Glucocorticoids and the preparation for life after birth: are there long-term consequences of the life insurance? Proc. Nutr. Soc. 57, 113–122 (1998).
Meyer, J. S. Early adrenalectomy stimulates subsequent growth and development of the rat brain. Exp. Neurol. 82, 432–446 (1983).
Bohn, M. C. in Neurobehavioural Teratology (ed. Yanai, J.) 365–387 (Elsevier, Amsterdam, 1984).
Effect of corticosteroids for fetal maturation on perinatal outcomes. NIH Consensus Development Panel on the Effect of Corticosteroids for Fetal Maturation on Perinatal Outcomes. JAMA 273, 413–418 (1995).
Roberts, D., Brown, J., Medley, N. & Dalziel, S. R. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst. Rev. 3, CD004454 (2017).
Sotiriadis, A. et al. Neurodevelopmental outcome after a single course of antenatal steroids in children born preterm: a systematic review and meta-analysis. Obstet. Gynecol. 125, 1385 (2015).
Ilg, L. et al. Persistent effects of antenatal synthetic glucocorticoids on endocrine stress reactivity from childhood to adolescence. J. Clin. Endocrinol. Metab. 104, 827–834 (2019).
Aghajafari, F. et al. Repeated doses of antenatal corticosteroids in animals: a systematic review. Am. J. Obstet. Gynecol. 186, 843–849 (2002).
Jobe, A. H. Animal models of antenatal corticosteroids: clinical implications. Clin. Obstet. Gynecol. 46, 174 (2003).
Moher, D., Liberati, A., Tetzlaff, J. & Altman, D. G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int. J. Surg. 8, 336–341 (2010).
Kemp, M. W., Schmidt, A. F., & Jobe, A. H. Optimizing antenatal corticosteroid therapy. Semin Fetal Neonatal Med. 24, 176–181 (2019).
Reagan-Shaw, S., Nihal, M., Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 22, 659–661 (2008).
Hooijmans, C. R. et al. SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 14, 43 (2014).
Hauser, J. et al. Effects of prenatal dexamethasone treatment on postnatal physical, endocrine, and social development in the common marmoset monkey. Endocrinology 148, 1813–1822 (2007).
Holson, R. R., Gough, B., Sullivan, P., Badger, T. & Sheehan, D. M. Prenatal dexamethasone or stress but not ACTH or corticosterone alter sexual behavior in male rats. Neurotoxicol. Teratol. 17, 393–401 (1995).
Brabham, T. et al. Effects of prenatal dexamethasone on spatial learning and response to stress is influenced by maternal factors. Am. J. Physiol. Regul. Integr. Comp. Physiol. 279, R1899–R1909 (2000).
Welberg, L. A., Seckl, J. R. & Holmes, M. C. Prenatal glucocorticoid programming of brain corticosteroid receptors and corticotrophin-releasing hormone: possible implications for behaviour. Neuroscience 104, 71–79 (2001).
Hougaard, K. S. et al. Prenatal stress may increase vulnerability to life events: comparison with the effects of prenatal dexamethasone. Brain Res. Dev. Brain Res. 159, 55–63 (2005).
Hauser, J., Feldon, J. & Pryce, C. R. Prenatal dexamethasone exposure, postnatal development, and adulthood prepulse inhibition and latent inhibition in Wistar rats. Behav. Brain Res. 175, 51–61 (2006).
Oliveira, M. et al. Induction of a hyperanxious state by antenatal dexamethasone: a case for less detrimental natural corticosteroids. Biol. Psychiatry 59, 844–852 (2006).
Shoener, J. A., Baig, R. & Page, K. C. Prenatal exposure to dexamethasone alters hippocampal drive on hypothalamic-pituitary-adrenal axis activity in adult male rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 290, R1366–R1373 (2006).
Nagano, M., Ozawa, H. & Suzuki, H. Prenatal dexamethasone exposure affects anxiety-like behaviour and neuroendocrine systems in an age-dependent manner. Neurosci. Res. 60, 364–371 (2008).
Hauser, J., Feldon, J. & Pryce, C. R. Direct and dam-mediated effects of prenatal dexamethasone on emotionality, cognition and HPA axis in adult Wistar rats. Horm. Behav. 56, 364–375 (2009).
Liu, W. et al. Swimming exercise ameliorates depression-like behaviors induced by prenatal exposure to glucocorticoids in rats. Neurosci. Lett. 524, 119–123 (2012).
Iwasa, T. et al. Prenatal exposure to glucocorticoids affects body weight, serum leptin levels, and hypothalamic neuropeptide-Y expression in pre-pubertal female rat offspring. Int. J. Dev. Neurosci. 36, 1–4 (2014).
Dong, W. et al. Low-functional programming of the CREB/BDNF/TrkB pathway mediates cognitive impairment in male offspring after prenatal dexamethasone exposure. Toxicol. Lett. 283, 1–12 (2018).
Liu, W. et al. OGT-related mitochondrial motility is associated with sex differences and exercise effects in depression induced by prenatal exposure to glucocorticoids. J. Affect. Disord. 226, 203–215 (2018).
Bruschettini, M. et al. Cognition- and anxiety-related behavior, synaptophysin and MAP2 immunoreactivity in the adult rat treated with a single course of antenatal betamethasone. Pediatr. Res. 60, 50–54 (2006).
Velísek, L. Prenatal exposure to betamethasone decreases anxiety in developing rats: hippocampal neuropeptide y as a target molecule. Neuropsychopharmacology 31, 2140–2149 (2006).
Bustamante, C. et al. Effects of a single course of prenatal betamethasone on dendritic development in dentate gyrus granular neurons and on spatial memory in rat offspring. Neuropediatrics 45, 354–361 (2014).
Pascual, R., Valencia, M., Larrea, S. & Bustamante, C. Single course of antenatal betamethasone produces delayed changes in morphology and calbindin-D28k expression in a rat’s cerebellar Purkinje cells. Acta Neurobiol. Exp. (Wars.) 74, 415–423 (2014).
Tsiarli, M. A. et al. Antenatal dexamethasone exposure differentially affects distinct cortical neural progenitor cells and triggers long-term changes in murine cerebral architecture and behavior. Transl. Psychiatry 7, e1153 (2017).
Shende, V. H., McArthur, S., Gillies, G. E. & Opacka-Juffry, J. Astroglial plasticity is implicated in hippocampal remodelling in adult rats exposed to antenatal dexamethasone. Neural Plast. 2015, 694347 (2015).
Lui, C.-C. et al. Effects of melatonin on prenatal dexamethasone-induced epigenetic alterations in hippocampal morphology and reelin and glutamic acid decarboxylase 67 levels. Dev. Neurosci. 37, 105–114 (2015).
Uno, H. et al. Neurotoxicity of glucocorticoids in the primate brain. Horm. Behav. 28, 336–348 (1994).
Dodic, M., Peers, A., Moritz, K., Hantzis, V. & Wintour, E. M. No evidence for HPA reset in adult sheep with high blood pressure due to short prenatal exposure to dexamethasone. Am. J. Physiol. Regul. Integr. Comp. Physiol. 282, R343–R350 (2002).
Banjanin, S., Kapoor, A. & Matthews, S. G. Prenatal glucocorticoid exposure alters hypothalamic-pituitary-adrenal function and blood pressure in mature male guinea pigs. J. Physiol. 558, 305–318 (2004).
Sloboda, D. M. et al. Expression of glucocorticoid receptor, mineralocorticoid receptor, and 11beta-hydroxysteroid dehydrogenase 1 and 2 in the fetal and postnatal ovine hippocampus: ontogeny and effects of prenatal glucocorticoid exposure. J. Endocrinol. 197, 213–220 (2008).
Li, S. et al. The effects of dexamethasone treatment in early gestation on hypothalamic-pituitary-adrenal responses and gene expression at 7 months of postnatal age in sheep. Reprod. Sci. 19, 260–270 (2012).
Diaz Heijtz, R., Fuchs, E., Feldon, J., Pryce, C. R. & Forssberg, H. Effects of antenatal dexamethasone treatment on glucocorticoid receptor and calcyon gene expression in the prefrontal cortex of neonatal and adult common marmoset monkeys. Behav. Brain Funct. 6, 18 (2010).
Sandman, C. A., Davis, E. P., Buss, C. & Glynn, L. M. Prenatal programming of human neurological function. Int. J. Pept. 2011, 837596 (2011).
Matthews, S. G. Antenatal glucocorticoids and programming of the developing CNS. Pediatr. Res. 47, 291–300 (2000).
Barrada, M. I., Blomquist, C. H. & Kotts, C. The effects of betamethasone on fetal development in the rabbit. Am. J. Obstet. Gynecol. 136, 234–238 (1980).
Frank, L. & Roberts, R. J. Effects of low-dose prenatal corticosteroid administration on the premature rat. Biol. Neonate 36, 1–9 (1979).
Muneoka, K. et al. Prenatal dexamethasone exposure alters brain monoamine metabolism and adrenocortical response in rat offspring. Am. J. Physiol. Regul. Integr. Comp. Physiol. 273, R1669–R1675 (1997).
McArthur, S., McHale, E., Dalley, J. W., Buckingham, J. C. & Gillies, G. E. Altered mesencephalic dopaminergic populations in adulthood as a consequence of brief perinatal glucocorticoid exposure. J. Neuroendocrinol. 17, 475–482 (2005).
Bruschettini, M., van den Hove, D. L. A., Gazzolo, D., Steinbusch, H. W. M. & Blanco, C. E. Lowering the dose of antenatal steroids: the effects of a single course of betamethasone on somatic growth and brain cell proliferation in the rat. Am. J. Obstet. Gynecol. 194, 1341–1346 (2006).
Noorlander, C. W., Visser, G. H. A., Ramakers, G. M. J., Nikkels, P. G. J. & Graan, P. N. Ede Prenatal corticosteroid exposure affects hippocampal plasticity and reduces lifespan. Dev. Neurobiol. 68, 237–246 (2008).
Noorlander, C. W. et al. Antenatal glucocorticoid treatment affects hippocampal development in mice. PLoS ONE 9, e85671 (2014).
Pascual, R., Valencia, M. & Bustamante, C. Effect of antenatal betamethasone administration on rat cerebellar expression of type la metabotropic glutamate receptors (mGluRla) and anxiety-like behavior in the elevated plus maze. Clin. Exp. Obstet. Gynecol. 43, 534–538 (2016).
Moss, T. J. M. et al. Effects into adulthood of single or repeated antenatal corticosteroids in sheep. Am. J. Obstet. Gynecol. 192, 146–152 (2005).
Weinstock, M. The long-term behavioural consequences of prenatal stress. Neurosci. Biobehav. Rev. 32, 1073–1086 (2008).
McArthur, S., Pienaar, I. S., Siddiqi, S. M. & Gillies, G. E. Sex-specific disruption of murine midbrain astrocytic and dopaminergic developmental trajectories following antenatal GC treatment. Brain Struct. Funct. 221, 2459–2475 (2016).
Korzhevskii, D. E., Gilerovich, E. G., Khozhai, L. I., Grigor’ev, I. P. & Otellin, V. A. Modification of histogenetic processes in rat nervous tissue after administration of dexamethasone during prenatal development. Neurosci. Behav. Physiol. 36, 537–539 (2006).
Leão, P. et al. Programming effects of antenatal dexamethasone in the developing mesolimbic pathways. Synapse 61, 40–49 (2007).
Oliveira, M. et al. Programming effects of antenatal corticosteroids exposure in male sexual behavior. J. Sex. Med. 8, 1965–1974 (2011).
Rodrigues, A. J. et al. Mechanisms of initiation and reversal of drug-seeking behavior induced by prenatal exposure to glucocorticoids. Mol. Psychiatry 17, 1295–1305 (2012).
Borges, S. et al. Dopaminergic modulation of affective and social deficits induced by prenatal glucocorticoid exposure. Neuropsychopharmacology 38, 2068–2079 (2013).
Hiroi, R., Carbone, D. L., Zuloaga, D. G., Bimonte-Nelson, H. A. & Handa, R. J. Sex-dependent programming effects of prenatal glucocorticoid treatment on the developing serotonin system and stress-related behaviors in adulthood. Neuroscience 320, 43–56 (2016).
Rosen, J. B. & Schulkin, J. From normal fear to pathological anxiety. Psychol. Rev. 105, 325–350 (1998).
Parnaudeau, S. et al. Glucocorticoid receptor gene inactivation in dopamine-innervated areas selectively decreases behavioral responses to amphetamine. Front. Behav. Neurosci. 8, 35 (2014).
Nagano, M., Liu, M., Inagaki, H., Kawada, T. & Suzuki, H. Early intervention with fluoxetine reverses abnormalities in the serotonergic system and behavior of rats exposed prenatally to dexamethasone. Neuropharmacology 63, 292–300 (2012).
Sidoryk-Wegrzynowicz, M., Wegrzynowicz, M., Lee, E., Bowman, A. B. & Aschner, M. Role of astrocytes in brain function and disease. Toxicol. Pathol. 39, 115–123 (2011).
Caetano, L. et al. Adenosine A2A receptor regulation of microglia morphological remodeling-gender bias in physiology and in a model of chronic anxiety. Mol. Psychiatry 22, 1035–1043 (2017).
Frahm, K. A., Handa, R. J. & Tobet, S. A. Embryonic exposure to dexamethasone affects nonneuronal cells in the adult paraventricular nucleus of the hypothalamus. J. Endocr. Soc. 2, 140–153 (2018).
De Kloet, E. R., Vreugdenhil, E., Oitzl, M. S. & Joëls, M. Brain corticosteroid receptor balance in health and disease. Endocr. Rev. 19, 269–301 (1998).
Jacobson, L. & Sapolsky, R. The role of the hippocampus in feedback regulation of the hypothalamic-pituitary-adrenocortical axis. Endocr. Rev. 12, 118–134 (1991).
Rayburn, W. F., Christensen, H. D. & Gonzalez, C. L. A placebo-controlled comparison between betamethasone and dexamethasone for fetal maturation: differences in neurobehavioral development of mice offspring. Am. J. Obstet. Gynecol. 176, 842–851 (1997).
Hauser, J. et al. Effects of prenatal dexamethasone treatment on physical growth, pituitary-adrenal hormones, and performance of motor, motivational, and cognitive tasks in juvenile and adolescent common marmoset monkeys. Endocrinology 149, 6343–6355 (2008).
Brownfoot, F. C., Gagliardi, D. I., Bain, E., Middleton, P. & Crowther, C. A. Different corticosteroids and regimens for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst. Rev. CD006764 (2013).
Ballard, P. L. & Ballard, R. A. Scientific basis and therapeutic regimens for use of antenatal glucocorticoids. Am. J. Obstet. Gynecol. 173, 254–262 (1995).
Lee, B. H., Stoll, B. J., McDonald, S. A., Higgins, R. D. & National Institute of Child Health and Human Development Neonatal Research Network. Adverse neonatal outcomes associated with antenatal dexamethasone versus antenatal betamethasone. Pediatrics 117, 1503–1510 (2006).
Pascual, R., Valencia, M. & Bustamante, C. Antenatal betamethasone produces protracted changes in anxiety-like behaviors and in the expression of microtubule-associated protein 2, brain-derived neurotrophic factor and the tyrosine kinase B receptor in the rat cerebellar cortex. Int. J. Dev. Neurosci. 43, 78–85 (2015).
Matthews, S. G. & Challis, J. R. G. Regulation of the hypothalamo-pituitary-adrenocortical axis in fetal sheep. Trends Endocrinol. Metab. 7, 239–246 (1996).
Matthews, S. G. Dynamic changes in glucocorticoid and mineralocorticoid receptor mRNA in the developing guinea pig brain. Dev. Brain Res. 107, 123–132 (1998).
Sapolsky, R. M. & Meaney, M. J. Maturation of the adrenocortical stress response: neuroendocrine control mechanisms and the stress hyporesponsive period. Brain Res. 396, 64–76 (1986).
Claman, H. N. Corticosteroids and lymphoid cells. N. Engl. J. Med. 287, 388–397 (1972).
Saccone, G. & Berghella, V. Antenatal corticosteroids for maturity of term or near term fetuses: systematic review and meta-analysis of randomized controlled trials. BMJ 355, i5044 (2016).
NICE. Preterm labour and birth | Guidance | Recommendations. https://www.nice.org.uk/guidance/ng25/chapter/Recommendations#maternal-corticosteroids [cited 19 May 2019].
Committee on Obstetric Practice. Committee Opinion No. 713: Antenatal Corticosteroid Therapy for Fetal Maturation. Obstet. Gynecol. 130, e102–e109 (2017).
Brown, R. W. et al. The ontogeny of 11 beta-hydroxysteroid dehydrogenase type 2 and mineralocorticoid receptor gene expression reveal intricate control of glucocorticoid action in development. Endocrinology 137, 794–797 (1996).
Murphy, V. E. & Clifton, V. L. Alterations in human placental 11β-hydroxysteroid dehydrogenase type 1 and 2 with gestational age and labour. Placenta 24, 739–744 (2003).
Smith, D. J., Joffe, J. M. & Heseltine, G. F. Modification of prenatal stress effects in rats by adrenalectomy, dexamethasone and chlorpromazine. Physiol. Behav. 15, 461–469 (1975).
Christensen, H. D., Gonzalez, C. L., Stewart, J. D. & Rayburn, W. F. Multiple courses of antenatal betamethasone and cognitive development of mice offspring. J. Matern. Fetal Med. 10, 269–276 (2001).
Burlet, G. et al. Antenatal glucocorticoids blunt the functioning of the hypothialamic-pituitary-adrenal axis of neonates and disturb some behaviors in juveniles. Neuroscience 133, 221–230 (2005).
McArthur, S., McHale, E. & Gillies, G. E. The size and distribution of midbrain dopaminergic populations are permanently altered by perinatal glucocorticoid exposure in a sex- region- and time-specific manner. Neuropsychopharmacology 32, 1462–1476 (2007).
Owen, D. & Matthews, S. G. Prenatal glucocorticoid exposure alters hypothalamic-pituitary-adrenal function in juvenile guinea pigs. J. Neuroendocrinol. 19, 172–180 (2007).
Setiawan, E., Jackson, M. F., MacDonald, J. F. & Matthews, S. G. Effects of repeated prenatal glucocorticoid exposure on long-term potentiation in the juvenile guinea-pig hippocampus. J. Physiol. 581, 1033–1042 (2007).
Hossain, A. et al. Prenatal dexamethasone impairs behavior and the activation of the BDNF exon IV promoter in the paraventricular nucleus in adult offspring. Endocrinology 149, 6356–6365 (2008).
Kleinhaus, K. et al. Effects of excessive glucocorticoid receptor stimulation during early gestation on psychomotor and social behavior in the rat. Dev. Psychobiol. 52, 121–132 (2010).
Neigh, G. N., Owens, M. J., Taylor, W. R. & Nemeroff, C. B. Changes in the vascular area fraction of the hippocampus and amygdala are induced by prenatal dexamethasone and/or adult stress. J. Cereb. Blood Flow. Metab. 30, 1100–1104 (2010).
Kjaer, S. L. et al. Influence of diurnal phase on startle response in adult rats exposed to dexamethasone in utero. Physiol. Behav. 102, 444–452 (2011).
Roque, S. et al. Interplay between depressive-like behavior and the immune system in an animal model of prenatal dexamethasone administration. Front. Behav. Neurosci. 5, 4 (2011).
Oliveira, M. et al. The bed nucleus of stria terminalis and the amygdala as targets of antenatal glucocorticoids: implications for fear and anxiety responses. Psychopharmacology (Berl.) 220, 443–453 (2012).
Zuloaga, D. G., Carbone, D. L. & Handa, R. J. Prenatal dexamethasone selectively decreases calretinin expression in the adult female lateral amygdala. Neurosci. Lett. 521, 109–114 (2012).
Virdee, K. et al. Antenatal glucocorticoid treatment induces adaptations in adult midbrain dopamine neurons, which underpin sexually dimorphic behavioral resilience. Neuropsychopharmacology 39, 339–350 (2014).
Frahm, K. A. & Tobet, S. A. Development of the blood-brain barrier within the paraventricular nucleus of the hypothalamus: influence of fetal glucocorticoid excess. Brain Struct. Funct. 220, 2225–2234 (2015).
Zeng, Y., Brydges, N. M., Wood, E. R., Drake, A. J. & Hall, J. Prenatal glucocorticoid exposure in rats: programming effects on stress reactivity and cognition in adult offspring. Stress 18, 353–361 (2015).
Virdee, K. et al. Counteractive effects of antenatal glucocorticoid treatment on D1 receptor modulation of spatial working memory. Psychopharmacology (Berl.) 233, 3751–3761 (2016).
Conti, M., Spulber, S., Raciti, M. & Ceccatelli, S. Depressive-like phenotype induced by prenatal dexamethasone in mice is reversed by desipramine. Neuropharmacology 126, 242–249 (2017).
Acknowledgements
J.L.v.d.M. is funded with support of the Erasmus+Programme of the European Union (Framework Agreement Number: 2013-0040). This publication reflects the views only of the author, and the Commission cannot be held responsible for any use which may be made of the information contained therein.
Author information
Authors and Affiliations
Contributions
All authors have contributed to the writing of this paper. J.L.v.d.M. and A.S. performed the search and extracted the data. J.T. and J.D. guided the results and merged and corrected the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
van der Merwe, J.L., Sacco, A., Toelen, J. et al. Long-term neuropathological and/or neurobehavioral effects of antenatal corticosteroid therapy in animal models: a systematic review. Pediatr Res 87, 1157–1170 (2020). https://doi.org/10.1038/s41390-019-0712-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-019-0712-1
This article is cited by
-
Value of preclinical systematic reviews and meta-analyses in pediatric research
Pediatric Research (2024)
-
Neurological implications of antenatal corticosteroids on late preterm and term infants: a scoping review
Pediatric Research (2022)
-
Antenatal corticosteroids and outcomes of small for gestational age infants born at 24–31 gestational weeks: a population-based propensity score matching analysis
Archives of Gynecology and Obstetrics (2022)