Abstract
Background
Indomethacin treatment for patent ductus arteriosus (PDA) is associated with acute kidney injury (AKI). Fenoldopam, a dopamine (DA) DA1-like receptor agonist dilates the renal vasculature and may preserve renal function during indomethacin treatment. However, limited information exists on DA receptor-mediated signaling in the ductus and fenoldopam may prevent ductus closure given its vasodilatory nature.
Methods
DA receptor expression in CD-1 mouse vessels was analyzed by qPCR and immunohistochemistry. Concentration−response curves were established using pressure myography. Pretreatment with SCH23390 (DA1-like receptor antagonist), phentolamine (α -adrenergic receptor antagonist) or indomethacin addressed mechanisms for DA-induced changes. Fenoldopam’s effects on postnatal ductus closure were evaluated in vivo.
Results
DA1 receptors were expressed equally in ductus and aorta. High-dose DA induced modest vasoconstriction under newborn O2 conditions. Phentolamine inhibited DA-induced constriction, while SCH23390 augmented constriction, consistent with a vasodilatory role for DA1 receptors. Despite this, fenoldopam had little effect on ductus tone nor indomethacin- or O2-induced constriction and did not impair postnatal closure in vivo.
Conclusion(s)
DA receptors are present in the ductus but have limited physiologic effects. DA-induced ductus vasoconstriction is mediated via α-adrenergic pathways. The absence of DA1-mediated impairment of ductus closure supports the study of potential role for fenoldopam during PDA treatment.
Similar content being viewed by others
Introduction
As many as 60–70% of newborns delivered prior to 28 weeks experience a patent ductus arteriosus (PDA) beyond the first few days of life.1 Although PDA-associated short- and long-term morbidity and the need for intervention remains debated, there is emerging evidence that PDA exposure into the second week of life may increase bronchopulmonary dysplasia.1 Safety concerns regarding pharmacologic closure of a PDA corresponds to the potential side effects caused by currently approved treatments, namely indomethacin and ibuprofen. While it has been recently suggested that indomethacin is superior to ibuprofen and acetaminophen in achieving ductus constriction, it poses significant risks for neonatal kidney toxicity.2,3 In 30% of preterm infants 24−32 weeks gestation, a single course of indomethacin resulted in oliguria or a significant decrease in urine output with an increase in serum creatinine.4 Even infants without frank oliguria often show significant decreases in urine output and increases in serum creatinine. To avoid fluid overload, the volume of parenteral nutrition provided is often reduced before or during indomethacin therapy, resulting in a reduction in energy, protein and carbohydrate intake.
Alleviating the risk and severity of renal impairment associated with indomethacin would remarkably reduce neonatal morbidity. However, no effective approach to attenuate this risk has been identified. Several studies support the use of low-dose dopamine to promote urine output in the preterm population, though data are insufficient due to variation in dosing and study population. A meta-analysis reviewing the effects of dopamine in indomethacin-treated infants found no evidence to support the use of dopamine to prevent renal dysfunction.5 In theory, low-dose dopamine results in renal vasodilation via activation of renal dopamine type 1 (DA1) receptors, avoiding the α1-adrenergic receptor-mediated renal vasoconstriction that occurs at higher concentrations of dopamine.
Fenoldopam ((-)-SKF-82526), a DA1-like receptor agonist, has the potential to dilate the renal vasculature since it avoids activating the vasoconstrictive adrenergic receptors typically seen at higher concentrations of dopamine. In critically ill adults and postoperative pediatric cardiac surgical patients, fenoldopam preserves renal function compared to placebo or dopamine.6,7 Studies in neonates are limited and suffer from retrospective design, small sample size, and heterogeneous study populations. However, in late gestation fetal sheep, an animal model with a pattern of renal development stage similar to humans, we showed that fenoldopam significantly increased glomerular filtration, urine flow, and sodium excretion.8 Demonstration of similar effects in the preterm infant would confer fenoldopam as an important therapeutic intervention in this population. While we are currently undertaking a randomized clinical trial to determine whether fenoldopam prevents renal dysfunction in preterm infants treated with indomethacin for PDA (NCT 02620761), little is known about the role of DA receptors in vasoreactivity of the ductus arteriosus. In the present study, we tested the hypothesis that dopamine signaling via DA receptors regulates ductus tone. Our objectives were to determine whether: (1) DA receptors are expressed and functional in the ductus; (2) fenoldopam alters vasomotor tone of the ductus; and (3) fenoldopam prevents postnatal ductus closure in vivo. Due to limitations in the availability of human ductus tissues for mechanistic research, we used the mouse as an animal model to evaluate the expression and activity of dopamine receptors in the ductus arteriosus.
Materials and methods
Animals
All experiments were conducted in accordance with National Institutes of Health Animal Care Standards and approved by the Institutional Animal Care and Use Committee at Vanderbilt University Medical Center. Adult female CD-1 mice were bred overnight to produce timed pregnancies. The presence of a vaginal plug was designated gestational day 1 (d1). On d19 (term gestation), pregnant mice were anesthetized by an intraperitoneal injection of 0.4 mL of 1.25% avertin (2,2,2-tribromoethanol in tert-amyl alcohol, Sigma-Aldrich, St. Louis, MO), followed by isoflurane inhalation, and cervical dislocation. Anesthetized fetuses were delivered via cesarean-section for tissue collection or were partially dissected to expose the thoracic cavity and then submerged in chilled, deoxygenated (95% N2, 5% CO2) modified Krebs (in mM; 109 NaCl, 4.7 KCl, 2.5 CaCl22H2O, 0.9 MgSO4, 1.0 KH2PO4, 11.1 glucose, 34 NaHCO3 (pH 7.3)).
Quantitative real-time PCR
Ductus and aorta samples from five representative fetuses from three different litters were excised on d19 of pregnancy. Samples were pooled by vessel type and litter. RNA was isolated using TRIzol Reagent (Life Technologies) and a bead homogenizer (BeadMill24, Fischer Scientific) and cDNA was generated using the SuperScript VILO cDNA Synthesis Kit (Invitrogen). Relative levels of gene expression were determined using TaqMan-based RT-qPCR and the Applied Biosystems StepOne Plus Real-Time PCR System with Step-One Software. Drd1 and Drd2 probes were used to determine DA1 and DA2 receptor expression levels, respectively. The housekeeping gene 18s was used as an internal control. Triplicate ΔΔCT values were generated and the fold change in expression was determined by dividing the ductus expression value by the aorta expression value, where aortic expression was set to 1.
Immunohistochemistry
The great arteries from d19 mouse fetuses were isolated en bloc, fixed with 4% paraformaldehyde, subjected to sucrose infiltration and cryosectioning (8 µm).
The immunohistochemistry methodological approach for identifying the localization of proteins encoded by Drd1 using an anti-DA1-receptor antibody (DRD1, Alomone Labs, ADR-001) and Drd2 using an anti-DA2-receptor antibody (DRD2, Alomone Labs, ADR-002) was performed as we previously reported for serotonin receptors in the mouse ductus arteriosus.9
Pressurized vessel myography
Ductus vessels from 7 to 9 fetuses representing at least three different litters were used for each myography study. The ductus was freshly isolated from d19 fetuses and vasoreactivity was evaluated using cannulated, pressurized vessel myography and computer-assisted videomicroscopy, as previously described.10,11,12,13 Briefly, the excised ductus was mounted in custom myography chambers (University of Vermont), then equilibrated for 40 min at 37 °C and 5 mmHg of distending pressure in modified, deoxygenated Krebs buffer. Chambers were placed on an inverted microscope equipped with a digital image capture system (IonOptix; Milton, MA) to record changes in the intraluminal diameter. Pressure was increased to 20 mmHg in 5-mmHg increments followed by exposure to 50 mM deoxy KCl (in mM: 64 NaCl, 50 KCl, 2.5 CaCl22H2O, 0.9 MgSO4, 1 KH2PO4, 11.1 glucose, 34 NaHCO3 (pH 7.3)) to determine vessel viability and peak contractility. Vessels were then changed from a flow-through system to a recirculating system (20 mL total volume) and allowed to re-equilibrate for 20 min. This lumen size was recorded as the resting lumen diameter or baseline (BL) for deoxygenated conditions. Changes in lumen diameter in response to increased concentrations (10–9 M to 10−4 M) of either dopamine HCl, fenoldopam HCl, SCH23390, the DA2 receptor antagonist L-741,626, or the α-adrenergic receptor antagonist phentolamine mesylate (all compounds from Tocris) were recorded and compared. Before each increase in drug concentration, lumen diameters were allowed time to achieve a new stable baseline (minimum of 20 min). For oxygen studies, vessels were changed from a recirculating system that was continuously aerated with deoxygenated gas (“fetal conditions”; pO2 ~38–42 Torr) to one aerated with 12% O2 (12% O2/5% CO2/balanced N2) (“newborn conditions”; pO2 ~115–120 Torr) for at least 60 min or until a new constricted baseline was achieved. This lumen size was recorded as the resting lumen diameter or baseline (BL) for oxygenated conditions. To eliminate the effects of endogenous prostaglandins, dopamine and fenoldopam dose response studies were repeated in the presence of indomethacin (10–5 M). In separate experiments, vessels were exposed to increasing concentrations of oxygen (Krebs buffer bubbled with either 0, 2, 5, 12, 21, or 95% O2/5% CO2/balanced N2) for at least 60 min per concentration in the continuous presence of 10−5 M fenoldopam. To determine if fenoldopam could reverse indomethacin-induced constriction, some vessels were pretreated with 10−5 M indomethacin (Sigma-Aldrich, St. Louis, MO) for 60 min followed by 10−5 M fenoldopam. At the end of every study, vessels were exposed to 50 mM KCl to verify vessel response and integrity.
In vivo evaluation of DA status
Mouse pups were delivered via cesarean-section on d19 then dried, stimulated, and placed onto a prewarmed surface set to37 °C. Thirty minutes after birth, littermates were randomly selected and treated with either control (saline) or drug (fenoldopam 1 mg/kg or PGE2 10 μg/kg) via intraperitoneal injection. Injections were then given hourly to provide a total of four injections. Pups underwent terminal anesthesia 30 min after the final injection via isoflurane inhalation and their chests were opened to determine the percent of ductus patency using a previously established visual scoring system.14
Statistical analysis
For myography studies, change in lumen diameter was plotted as percent change compared to baseline diameter at resting tone. Drug doses represent the cumulative final molar concentration in the recirculating system. Best-fit curves and sigmoidal approximation were analyzed for each dataset (Prism 6, Graphpad Software, La Jolla, CA). Either a paired t test (gene expression studies) or ANOVA with Bonferroni multiple comparison test (vessel studies) was used to determine statistical significance. The effects of oxygen condition or drug concentration were analyzed by one-way ANOVA; response curves between two different drugs or conditions were compared by two-way ANOVA. All data are represented as mean ± SEM. p values < 0.05 were considered significant.
Results
Dopamine receptors are expressed and functional in the isolated ductus
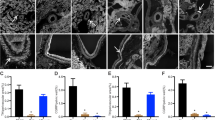
Quantitative RT-PCR demonstrated DA receptors are expressed in both the ductus and the ascending aorta of fetal mice (Fig. 1a), with equal expression of the DA1 (aorta, 0.986 ± 0.152; ductus, 0.7912 ± 0.172) but significantly higher expression of DA2 receptors in the ductus (aorta, 0.944 ± 0.133; ductus, 3.61 ± 0.662; p < 0.05). Immunostaining showed localization of DA1 receptors in the muscular media and adventitia and DA2 receptors in the intimal and adventitial layers of the ductus (Fig. 1c, d).
a RT-qPCR indicates the presence of DA1 and DA2 dopamine receptors in the day 19 mouse embryo ductus arteriosus (d19 DA) compared to aorta (d19 Aorta) (15 representative offspring from 3 litters). Isolation and immunostaining of term gestation outflow tracts (b) showed localization of DA1 receptor protein (DRD1) in the muscular media and adventitia (arrows) (c) and localization of DA2 receptor protein (DRD2) in the intimal (endothelial) and adventitial layers (d) of the ductus (arrows). e The isolated ductus displayed significant dopamine-induced vasoconstriction compared to baseline diameter under deoxy (“fetal”; open circle; n = 9) and 12% (“newborn”; filled circle; n = 7) oxygen conditions. f Term vessels under newborn oxygen conditions were pretreated with different inhibitors: 10−6 M SCH23390 (open circle; n = 8), L-741,626 (open square; n = 9), or phentolamine (filled triangle; n = 9), compared to untreated vessels (12% O2; filled circle; n = 7). All pretreated vessels had significant change in ductus diameter at Emax compared to resting baseline (BL) dimensions (*) and were significantly different from vessels that received no pretreatment (†p < 0.0001, two-way ANOVA). Data shown are mean ± SEM; p < 0.05 (*) was considered significant. AO aorta, BPA branch pulmonary artery, DA ductus arteriosus, MPA main pulmonary artery.
To determine whether these receptors are functional, isolated vessels were exposed to increasing concentrations of DA (10−9–10−4 M). Under fetal oxygen conditions (deoxy), DA produced a significant concentration-dependent constriction (p < 0.01), whereas maximum constriction from resting baseline (38%) occurred under newborn O2 conditions (Fig. 1e). These responses were unaffected by pretreatment with indomethacin (Supp. Fig. 1). Our observations coincide with previous studies, which show that higher concentrations of DA produce greater vasoconstrictive effects due to the activation of adrenergic receptors rather than the stimulation of DA receptors.15,16 Therefore, to examine the potential mechanisms by which high-dose DA induces ductus constriction, isolated vessels were first exposed to newborn oxygen conditions before receiving pretreatment with 10−6 M of either a DA1-like receptor antagonist (SCH23390), a DA2 receptor antagonist (L-741,626), or an α-adrenergic receptor antagonist (phentolamine), followed by exposure to increasing concentrations of DA (Fig. 1f).
Pretreatment of the isolated ductus with the SCH23390 produced a significant increase in DA-induced constriction, reaching a maximum of 55% decrease in lumen diameter from baseline (p < 0.0001). This was significantly greater than vessels that were not pretreated (Emax 38%; p < 0.0001), consistent with a vasodilatory role for DA1-like receptors in the ductus (Fig. 1f). On the other hand, pretreatment of the ductus with the DA2 receptor antagonist L-741,626 or phentolamine, abolished the observed concentration-dependent constriction at lower concentrations of DA and instead resulted in ductus dilation, reaching a maximum of 8% (L-741,626; p < 0.001) and 17% (phentolamine; p < 0.001) greater lumen diameter than at basal tone. Furthermore, pretreatment with L-741,626 and phentolamine significantly altered DA-induced effects on the ductus when compared to vessels solely treated with DA (p < 0.01 and p < 0.0001, respectively). These findings are consistent with DA-induced vasoconstriction via DA2 and α-adrenergic receptors at higher concentrations.
Fenoldopam (a DA1-like receptor agonist) has an insignificant effect on ductus tone
Fenoldopam, a DA1-like receptor agonist, has been previously reported to relax isolated arteries in a concentration-dependent manner.17,18 Under fetal oxygen conditions, increasing concentrations of fenoldopam had either no effect on ductus tone or exhibited a slight uptrend in vessel diameter at the highest concentration. On the other hand, vessels under newborn oxygen conditions displayed a minor concentration-dependent constriction (Fig. 2a, b), although neither effects were statistically significant compared to baseline lumen diameter. A significant difference was noted when the two oxygen conditions were compared (p < 0.0001). These responses were unaffected by pretreatment with indomethacin (Supp. Fig. 2).
a Representative tracing of changes in ductus lumen diameter in response to initial KCl stimuli, shift in oxygen conditions (12%), and progressive drug exposure. b Cumulative concentration response curves show that the ex vivo ductus exposed to increasing concentrations of fenoldopam have nonsignificant changes in lumen diameter under deoxy (“fetal”; open circle; n = 8) or 12% (“newborn”; filled circle; n = 9) oxygen conditions in comparison to baseline (BL) diameter. A small but significant difference was noted between the two oxygen conditions (†p < 0.0001, two-way ANOVA). Data shown are mean ± SEM.
Fenoldopam does not affect the ductus’ response to oxygen
To determine whether fenoldopam treatment can interfere with the ability of the ductus to sense and respond to oxygen by any of the proposed mechanisms for oxygen-induced ductus closure,19 vessels in deoxygenated conditions were abruptly exposed to 12% O2 to simulate the rise in oxygen tension at birth. Acute exposure constricted the ex vivo ductus from resting tone by 54% (Fig. 3a). Vessels pretreated with 10−5 M fenoldopam prior to 12% O2 exposure constricted to approximately the same degree (48%) (p > 0.05) (Fig. 3b), suggesting that fenoldopam does not impair O2-induced constriction of the ductus in newborn oxygen conditions. This effect was similar in the presence or absence of indomethacin (10−5 M) (Suppl. Fig. 3).
a Representative tracing of ductus lumen diameter changes in response to 12% O2 in the absence or presence of 10−5 M fenoldopam. b Under newborn oxygen conditions (12%), the ductus significantly constricted from resting baseline, regardless of fenoldopam exposure (54% vs. 48%; n = 7). c In separate experiments, progressive exposure to increasing oxygen in the organ bath induced significant ductus constriction (Krebs buffer bubbled with 0–95% O2). Oxygen-induced ductus constriction in the presence of 10−5 M fenoldopam (open circle; n = 9) was not significantly different than constriction without drug (filled circle; n = 9). Data shown are mean ± SEM; p value < 0.05 (*) was considered significant compared to baseline (BL) diameter in 0% oxygen conditions.
To further determine whether fenoldopam could affect O2-induced ductus constriction, a separate set of vessels were first pretreated with 10−5 M fenoldopam and then exposed to increasing concentrations of oxygen (0, 2, 5, 12, 21, and 95%) while continuously in the presence of fenoldopam (Fig. 3c). At concentrations below 12%, there was a decreased trend in oxygen-induced ductus constriction (nonsignificant), whereas at oxygen concentrations above 12%, an increased trend in ductus constriction was observed (nonsignificant). At 12% oxygen, there was no difference in ductus tone, corresponding to findings in on−off exposure studies (Fig. 3a, b). Although oxygen exposure induced significant constriction compared to baseline lumen diameter under each condition (p < 0.0001), there was no difference between untreated vessels and fenoldopam pretreated vessels.
Fenoldopam does not inhibit indomethacin-induced constriction of the ductus arteriosus
To determine whether fenoldopam’s vasodilatory properties might impair indomethacin-induced ductus constriction, the isolated ductus was pretreated with 10−5 M indomethacin followed by 10−5 M fenoldopam under both newborn (Fig. 4a, b) and fetal (Fig. 4c) oxygen conditions. The ability of indomethacin to constrict the ex vivo ductus was significantly lower in fetal oxygen conditions, but in both cases, fenoldopam did not impair indomethacin-induced constriction of the ductus (p > 0.05).
a Representative tracing of ductus lumen diameter changes in response to 10−5 M fenoldopam in the presence of both newborn oxygen conditions (12%) and 10−5 M indomethacin. b Constriction of the isolated ductus after exposure to newborn oxygen conditions (12%) and treatment with indomethacin was no different than similarly treated vessels in the presence of fenoldopam (n = 8). c In separate experiments, fenoldopam had no effect on indomethacin-induced constriction under fetal oxygen conditions (n = 9). Data shown are mean ± SEM.
In vivo exposure to fenoldopam does not prevent postnatal closure of the ductus
Treatment with fenoldopam has the potential to produce vasodilation in any vascular bed that is rich in DA1-like receptors. Because our data show that the ductus expresses these receptors, we evaluated whether fenoldopam could impair postnatal closure of the ductus in vivo. Compared to saline injected newborns, offspring treated with fenoldopam had no impairment in ductus closure after 4 h in room air. By comparison, newborn pups treated with PGE2 (as a positive control) demonstrated the presence of PDA with a significant decrease in the frequency of postnatal ductus closure (Fig. 5a, b).
Newborn littermates reared in room air were injected with either saline (control), fenoldopam, or PGE2. a Representative images show ductus closure at 4 h of age in a fenoldopam-injected animal and persistent patency of the DA in a PGE2-treated littermate. AO aorta, PA pulmonary artery. Arrow indicated ductus arteriosus. b Frequency distribution showing the percent patency of the ductus arteriosus in newborn mice exposed to control (saline; black bars; 14 representative pups from 5 litters), fenoldopam (1 mg/kg; white bars; 18 representative pups from 2 litters), or PGE2 (10 μg/kg; hatched bars; 27 representative pups from 6 litters). Percent total represents all animals studies (i.e. cumulative percent total for each group equals 100%). All control- and fenoldopam-treated mice had complete ductus closure by 4 h of age, whereas 89% of pups receiving PGE2 treatment displayed some degree of patency, ranging from 10 to 100% patent.
Discussion
Dopamine is one of the most commonly prescribed drugs for extremely low birth weight infants in the NICU, often being used for the treatment of systemic hypotension associated with PDA.20 Additionally, dopamine has been used to induce renal vasodilation and maintain urine output in indomethacin-treated infants.21 However, little is known regarding the role of dopamine and selective dopamine receptors on vasoreactivity of the ductus arteriosus. Using RT-qPCR and immunostaining techniques, we demonstrated the presence of DA1 and DA2 receptors within the term mouse ductus arteriosus. Under newborn O2 conditions, DA induced modest ductus vasoconstriction at concentrations (10−6–10−4 M) that typically exceed those obtained during systemic dopamine infusion.22 Alpha-adrenergic receptor blockade with phentolamine inhibited DA-induced ductus constriction, suggesting the vasoconstrictive effects of DA are mediated through these receptors. A vasoconstrictive effect of dopamine on the isolated ductus is not surprising given its known pharmacologic properties on other vascular tissues. At low concentrations, dopamine selectively binds to Gαs protein-coupled vascular DA1 receptors, activating adenylate cyclase activity and raising intracellular cAMP concentrations, leading to vasodilation. At higher concentrations, dopamine activates vascular α1-adrenergic receptors resulting in vasoconstriction and increased regional arterial resistance. Coadministration of phentolamine, an α-adrenergic receptor antagonist, attenuated this response in the mouse ductus, suggesting similar mechanisms are in place in this tissue.
Similar to our previous studies in this model, we found differential effects of drug exposure depending upon the oxygen concentration (fetal vs. newborn conditions). The response of the ductus to increased oxygen tension is complex, accentuating contractile responses and modulating vasodilatory responses to various stimuli via multiple signaling pathways.
In our mouse model, we have previously demonstrated oxygen-dependent differences (fetal vs. newborn oxygen conditions) to a number of vasoactive agents and that these differences are, in part, related to isoprostane generation.23 The generation of other types of reactive oxygen species as well as direct effects of increased oxygen tension on numerous factors mediating ductal tone has been demonstrated by other investigators.19,24
The effects of systemic infusion of dopamine on ductus patency in preterm infants is unknown. In hypotensive infants with PDA, dopamine infusion (13 ± 5 µg/kg/min) increased systemic and pulmonary artery pressures.25 The pulmonary/systemic mean arterial pressure ratio increased in nine infants, whereas it remained unchanged or decreased in the other nine infants. Unfortunately, the impact of dopamine infusion on ductus vasoreactivity was not evaluated. However, in a small trial of infants designed to determine if concomitant dopamine infusion impacted ductus closure rates, no impact of low dose (2 µg/kg/min) or high dose (5 µg/kg/min) was found.26 To our knowledge, the effect of dopamine on the isolated ductus arteriosus has not been previously studied in vivo or in vitro.
Five subtypes of dopamine receptors (D1, D2, D3, D4 and D5 receptors) have been described that mediate the physiological functions of dopamine in the brain, kidney, gastrointestinal and cardiovascular system. As members of the GPCR superfamily, DA receptors have a canonical seven-transmembrane structure and signal through both G-protein-dependent and -independent mechanisms.27 DA1-like and DA2-like receptors have been localized in a large number of arterial vascular beds.15 Previous studies using various adult vessels have shown stimulation of vascular DA1-like receptors causes direct vasodilation via activation of adenylate cyclase activity and increasing intracellular cAMP concentrations, while stimulation of vascular DA2-like receptors results in indirect vasodilatation from inhibition of norepinephrine release and sympathetic vasoconstrictor tone.28,29
DA1-like receptors are ubiquitously expressed in the systemic arterial system, localized primarily to the tunica media of the aorta, carotid, vertebral, mesenteric and renal arteries and multiple layers of the pulmonary artery.15,30,31 In spontaneously hypertensive rats, fenoldopam produced dose-dependent decreases in arterial pressure while significantly increasing mesenteric and renal blood flows.32 Pretreatment with the DA1-like receptor antagonist SCH23390 significantly attenuated these effects, suggesting that fenoldopam-mediated vasodilation resulted from stimulation of vascular DA1 receptors. In vitro, fenoldopam relaxed preconstricted human arteries from brachial, cerebral, cervical, colic, coronary, lumbar, pulmonary, renal and splenic sites.28 The effects of fenoldopam were not endothelium dependent but were antagonized by the DA1-like receptor antagonist, SCH23390. In adult humans, fenoldopam also results in dose-dependent decreases in systolic and diastolic blood pressure. In the pediatric population (1 month–12 years of age), fenoldopam decreased mean blood pressure at doses as low as 0.2 μg/kg/min, with a maximal effect at 0.8 μg/kg/min.33
Little is known regarding the activity of DA1-like receptors in development. In human umbilical arteries, fenoldopam induces vasoconstriction at supratherapeutic concentrations. This effect can be blocked by the irreversible alpha-adrenergic receptor antagonist phenoxybenzamine but not the reversible antagonist phentolamine.34 We previously examined the effects of systemic fenoldopam infusion in preterm and near-term fetal sheep. In contrast to results in postnatal animals and humans, fenoldopam increased mean arterial blood pressure in a dose-dependent manner via unknown mechanisms.8 Additionally, fenoldopam had no effect on renal blood flow velocity, but increased urine volume and sodium excretion in near-term, but not preterm sheep. This latter finding suggests that renal DA1 receptors and/or coupled second messenger systems are developmentally regulated.
We determined that DA1 receptors were expressed at equal levels in the ductus and ascending aorta of fetal mice, whereas DA2 receptors were more highly expressed in the ductus. Interestingly, exposure to the DA2 receptor antagonist L-741,626 attenuated the vasoconstrictive effects of dopamine, which is surprising given the purported role of DA2 in suppressing norepinephrine release, and thus functioning in a vasodilator capacity.35 Deletion of the Drd2 gene that encodes for the DA2 receptor results in hypertension in mice.36 Furthermore, in porcine carotid arteries, activation of DA2 increased eNOS and iNOS expression in VSM and endothelium which in turn would result in vasodilation.37 These contrasting findings suggest further study of the DA2 receptor in the fetal mouse ductus is needed.
In contrast, we found the DA1-like receptor antagonist SCH23390 augmented DA-induced ductus constriction, consistent with a vasodilating role for the DA1 receptor. Moreover, fenoldopam induced minor ductus dilation in fetal O2 conditions and minor constriction in newborn O2 conditions while having no appreciable effect on indomethacin- or O2-induced ductus constriction. Finally, systemic administration of fenoldopam demonstrated no impairment of postnatal closure in vivo. Taken together, our findings collectively suggest that: (1) DA1-like receptor activation has limited effects on ductus vasoreactivity and (2) the clinical use of DA1 agonists to attenuate the risk of renal injury will not negatively impact natural or pharmacological ductus closure.
Our study is not without limitations. First, we did not examine the entire family of dopamine receptors. Additional studies are needed to fully understand specific roles of DA1-like and DA2-like receptors within the ductus. Second, our model system does not allow continuous infusions of drugs for in vivo studies. Rather, we are limited to repeated intraperitoneal dosing, making drug dosing problematic. For example, our dose of fenoldopam was chosen to be approximately tenfold greater than that shown to be active when delivered intravenously.38 However, data regarding the biological activity of our chosen dose in newborn mice are lacking. Finally, while our long-term clinical goal is to study the efficacy of fenoldopam in attenuating the risk of kidney injury during PDA treatment, we did not evaluate the expression of dopamine receptors in the newborn mouse kidney. While renal vascular and nephron dopamine receptor localization has been explored in the adult, developmental expression has not been explored.39 A comprehensive evaluation is beyond the scope of this work. Additionally, studies in late gestation fetal have previously demonstrated fenoldopam increases glomerular filtration rate, urinary flow rate and urinary sodium excretion.8
Experience with fenoldopam in neonates is limited. In a retrospective review of 22 neonates administered fenoldopam to treat oliguria, no significant changes in urine output, serum creatinine, or electrolytes were found.40 In contrast, Costello et al. reported in a retrospective cohort that use of fenoldopam in postoperative neonates with insufficient response to diuretics increased urine output.6 Clearly additional studies are needed to determine if fenoldopam may be of therapeutic benefit in the neonatal population. The results of the present study, in which selective DA1 receptor activation had little or no effect on ex vivo ductus constriction nor in vivo closure, provide reassurance that concomitant use of dopamine or fenoldopam should not negatively impact rates of ductus closure with indomethacin. Our ongoing clinical trial of fenoldopam to prevent AKI in preterm infants receiving indomethacin will help in determining if there is a place for this agent in the limited pharmaceutical armamentarium available to preterm infants.
References
Gillam-Krakauer, M. & Reese, J. Diagnosis and management of patent ductus arteriosus. Neoreviews 19, e394–e402 (2018).
Clyman, R. I. et al. PDA-TOLERATE Trial: an exploratory randomized controlled trial of treatment of moderate-to-large patent ductus arteriosus at 1 week of age. J. Pediatr. 205, 41–48.e46 (2019).
Shaffer, C. L. et al. Effect of age and birth weight on indomethacin pharmacodynamics in neonates treated for patent ductus arteriosus. Crit. Care Med. 30, 343–348 (2002).
Lee, J. et al. Randomized trial of prolonged low-dose versus conventional-dose indomethacin for treating patent ductus arteriosus in very low birth weight infants. Pediatrics 112, 345–350 (2003).
Barrington K. J., Brion L. P. Dopamine versus no treatment to prevent renal dysfunction in indomethacin‐treated preterm newborn infants. Cochrane Database Syst. Rev. 2002, CD003213. https://doi.org/10.1002/14651858.CD003213.
Costello, J. M. et al. Initial experience with fenoldopam after cardiac surgery in neonates with an insufficient response to conventional diuretics. Pediatr. Crit. Care Med. 7, 28–33 (2006).
Zangrillo, A. et al. Fenoldopam and acute renal failure in cardiac surgery: a meta-analysis of randomized placebo-controlled trials. J. Cardiothorac. Vasc. Anesth. 26, 407–413 (2012).
Segar, J. L., Smith, F. G., Guillery, E. N., Jose, P. A. & Robillard, J. E. Ontogeny of renal response to specific dopamine DA1-receptor stimulation in sheep. Am. J. Physiol. 263, R868–R873 (1992).
Hooper, C. W. et al. Selective serotonin reuptake inhibitor exposure constricts the mouse ductus arteriosus in utero. Am. J. Physiol. Heart Circ. Physiol. 311, H572–H581 (2016).
Reese, J. et al. Regulation of the fetal mouse ductus arteriosus is dependent on interaction of nitric oxide and COX enzymes in the ductal wall. Prostaglandins Other Lipid Mediat. 88, 89–96 (2009).
Cotton, R. B. et al. Cimetidine-associated patent ductus arteriosus is mediated via a cytochrome P450 mechanism independent of H2 receptor antagonism. J. Mol. Cell Cardiol. 59, 86–94 (2013).
Pfaltzgraff, E. R. et al. Embryonic domains of the aorta derived from diverse origins exhibit distinct properties that converge into a common phenotype in the adult. J. Mol. Cell Cardiol. 69, 88–96 (2014).
Shelton, E. L. et al. Transcriptional profiling reveals ductus arteriosus-specific genes that regulate vascular tone. Physiol. Genomics 46, 457–466 (2014).
Vucovich, M. M. et al. Aminoglycoside-mediated relaxation of the ductus arteriosus in sepsis-associated PDA. Am. J. Physiol. Heart Circ. Physiol. 307, H732–H740 (2014).
Bucolo, C., Leggio, G. M., Drago, F. & Salomone Dopamine outside the brain: the eye, cardiovascular system and endocrine pancreas. Pharmacol. Therapeutics 9, 107392 (2019).
Shepperson, N. B., Duval, N. & Langer, S. Z. Dopamine decreases mesenteric blood flow in the anaesthetised dog through the stimulation of postsynaptic alpha 2-adrenoceptors. Eur. J. Pharm. 81, 627–635 (1982).
Hughes, A. D. & Sever, P. S. The action of dopamine and vascular dopamine (DA1) receptor agonists on human isolated subcutaneous and omental small arteries. Br. J. Pharm. 97, 950–956 (1989).
Kohli, J. D., Glock, D. & Goldberg, L. I. Relative DA1-dopamine-receptor agonist and alpha-adrenoceptor antagonist activity of fenoldopam in the anesthetized dog. J. Cardiovasc. Pharm. 11, 123–126 (1988).
Stoller, J. Z., Demauro, S. B., Dagle, J. M. & Reese, J. Current perspectives on pathobiology of the ductus arteriosus. J. Clin. Exp. Cardiol. 8, S8–001 (2012).
Hsieh, E. M. et al. Medication use in the neonatal intensive care unit. Am. J. Perinatol. 31, 811–821 (2014).
Seri, I., Tulassay, T., Kiszel, J. & Csomor, S. The use of dopamine for the prevention of the renal side effects of indomethacin in premature infants with patent ductus arteriosus. Int. J. Pediatr. Nephrol. 5, 209–214 (1984).
Johnston, A. J. et al. Pharmacokinetics and pharmacodynamics of dopamine and norepinephrine in critically ill head-injured patients. Intensive Care Med. 30, 45–50 (2004).
Chen, J. X. et al. Isoprostanes as physiological mediators of transition to newborn life: novel mechanisms regulating patency of the term and preterm ductus arteriosus. Pediatr. Res. 72, 122–128 (2012).
Hung, Y.-C., Yeh, J.-L. & Hsu, J.-H. Molecular mechanisms for regulating postnatal ductus arteriosus closure. Int. J. Mol. Sci. 19, E1861 (2018).
Liet, J. M. et al. Dopamine effects on pulmonary artery pressure in hypotensive preterm infants with patent ductus arteriosus. J. Pediatr. 140, 373–375 (2002).
Fajardo, C. A., Whyte, R. K. & Steele, B. T. Effect of dopamine on failure of indomethacin to close the patent ductus arteriosus. J. Pediatr. 121, 771–775 (1992).
Beaulieu, J. M., Espinoza, S. & Gainetdinov, R. R. Dopamine receptors—IUPHAR Review 13. Br. J. Pharm. 172, 1–23 (2015).
Hughes, A. D. & Sever, P. S. Action of fenoldopam, a selective dopamine (DA1) receptor agonist, on isolated human arteries. Blood Vessels 26, 119–127 (1989).
Lokhandwala, M. F. & Barrett, R. J. Dopamine receptor agonists in cardiovascular therapy. Drug Dev. Res. 3, 299–310 (1983).
Kim, M. O. et al. Localization of dopamine D1 and D2 receptor mRNAs in the rat systemic and pulmonary vasculatures. Mol. Cells 9, 417–421 (1999).
Amenta, F. et al. Localization of dopamine receptor subtypes in systemic arteries. Clin. Exp. Hypertens. 22, 277–288 (2000).
Lappe, R. W., Todt, J. A. & Wendt, R. L. Effects of fenoldopam on regional vascular resistance in conscious spontaneously hypertensive rats. J. Pharm. Exp. Ther. 236, 187–191 (1986).
Hammer, G. B., Verghese, S. T., Drover, D. R., Yaster, M. & Tobin, J. R. Pharmacokinetics and pharmacodynamics of fenoldopam mesylate for blood pressure control in pediatric patients. BMC Anesthesiol. 8, 6 (2008).
Sato, N. et al. The vasodilatory effects of hydralazine, nicardipine, nitroglycerin, and fenoldopam in the human umbilical artery. Anesth. Analg. 96, 539–544 (2003).
Taylor, A. A. et al. Activation of peripheral dopamine presynaptic receptors lowers blood pressure and heart rate in dogs. Hypertension 5, 226–234 (1983).
Li, X. X. et al. Adrenergic and endothelin B receptor-dependent hypertension in dopamine receptor type-2 knockout mice. Hypertension 38, 303–308 (2001).
Pyne-Geithman, G. J., Caudell, D. N., Cooper, M., Clark, J. F. & Shutter, L. A. Dopamine D2-receptor-mediated increase in vascular and endothelial NOS activity ameliorates cerebral vasospasm after subarachnoid hemorrhage in vitro. Neurocrit. Care 10, 225–231 (2009).
Escano, C. S. et al. Renal dopaminergic defect in C57Bl/6J mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 297, R1660–R1669 (2009).
Jose, P. A. et al. The renal dopamine receptors. J. Am. Soc. Nephrol. 2, 1265–1278 (1992).
Yoder, S. E. & Yoder, B. A. An evaluation of off-label fenoldopam use in the neonatal intensive care unit. Am. J. Perinatol. 26, 745–750 (2009).
Acknowledgements
This study was supported in part by NIH grants HL132805 (E.L.S.), HL109199 (J.R.), HL128386 (J.R., E.L.S.), and DK113073 (J.L.S.).
Author information
Authors and Affiliations
Contributions
S.L.C., M.H., N.B., R.L.S., M.T.Y., C.D.B., E.L.S., J.R., and J.L.S. made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; E.L.S., J.R., and J.L.S. participated in drafting and critical revisions for important intellectual content; J.R. and J.L.S. had final approval of the version to be published.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Crockett, S.L., Harris, M., Boatwright, N. et al. Role of dopamine and selective dopamine receptor agonists on mouse ductus arteriosus tone and responsiveness. Pediatr Res 87, 991–997 (2020). https://doi.org/10.1038/s41390-019-0716-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-019-0716-x