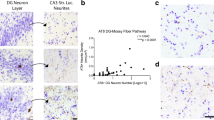
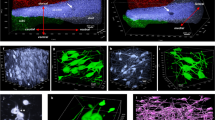
Abstract
In Alzheimer’s disease (AD), Tau and Aβ aggregates involve sequentially connected regions, sometimes distantly separated. These alterations were studied in the pillar of the fornix (PoF), an axonal tract, to analyse the role of axons in their propagation. The PoF axons mainly originate from the subicular neurons and project to the mamillary body. Forty-seven post-mortem cases at various Braak stages (Tau) and Thal phases (Aβ) were analysed by immunohistochemistry. The distribution of the lesions showed that the subiculum was affected before the mamillary body, but neither Tau aggregation nor Aβ deposition was consistently first. The subiculum and the mamillary body contained Gallyas positive neurofibrillary tangles, immunolabelled by AT8, TG3, PHF1, Alz50 and C3 Tau antibodies. In the PoF, only thin and fragmented threads were observed, exclusively in the cases with neurofibrillary tangles in the subiculum. The threads were made of Gallyas negative, AT8 and TG3 positive Tau. They were intra-axonal and devoid of paired helical filaments at electron microscopy. We tested PoF homogenates containing Tau AT8 positive axons in a Tau P301S biosensor HEK cell line and found a seeding activity. There was no Aβ immunoreactivity detected in the PoF. We could follow microcryodissected AT8 positive axons entering the mamillary body; contacts between Tau positive endings and Aβ positive diffuse or focal deposits were observed in CLARITY-cleared mamillary body. In conclusion, we show that non-fibrillary, hyperphosphorylated Tau is transported by the axons of the PoF from the subiculum to the mamillary body and has a seeding activity. Either Tau aggregation or Aβ accumulation may occur first in this system: this inconstant order is incompatible with a cause-and-effects relationship. However, both pathologies were correlated and intimately associated, indicating an interaction of the two processes, once initiated.










Similar content being viewed by others
References
Ando K, Laborde Q, Lazar A, Godefroy D, Youssef I, Amar M et al (2014) Inside Alzheimer brain with CLARITY: senile plaques, neurofibrillary tangles and axons in 3-D. Acta Neuropathol 128:457–459. https://doi.org/10.1007/s00401-014-1322-y
Braak H, Braak E (1991) Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 82:239–259
Braak H, Braak E (1997) Frequency of stages of Alzheimer-related lesions in different age categories. Neurobiol Aging 18:351–357
Braak H, Del Tredici K (2013) Amyloid-β may be released from non-junctional varicosities of axons generated from abnormal tau-containing brainstem nuclei in sporadic Alzheimer’s disease: a hypothesis. Acta Neuropathol 126:303–306. https://doi.org/10.1007/s00401-013-1153-2
Braak H, Braak E, Grundke-Iqbal I, Iqbal K (1986) Occurrence of neuropil threads in the senile human brain and in Alzheimer’s disease: a third location of paired helical filaments outside of neurofibrillary tangles and neuritic plaques. Neurosci Lett 65:351–355
Braak H, Del Tredici K, Rüb U, De Vos RAI, Jansen Steur ENH, Braak E (2003) Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging 24:197–211. https://doi.org/10.1016/S0197-4580(02)00065-9
Brion JP, Passareiro H, Nunez J, Flament-Durand J (1985) Mise en évidence immunologique de la protéine Tau au niveau des lésions de dégénérescence neurofibrillaire de la maladie d’Alzheimer. Arch Biol (Bruxelles) 95:229–235
Calafate S, Buist A, Miskiewicz K, Vijayan V, Daneels G, de Strooper B et al (2015) Synaptic contacts enhance cell-to-cell tau pathology propagation. Cell Rep 11:1176–1183. https://doi.org/10.1016/j.celrep.2015.04.043
Cali I, Cohen ML, Haik S, Parchi P, Giaccone G, Collins SJ et al (2018) Iatrogenic Creutzfeldt–Jakob disease with Amyloid-β pathology: an international study. Acta Neuropathol Commun 6:5. https://doi.org/10.1186/s40478-017-0503-z
Clavaguera F, Bolmont T, Crowther RA, Abramowski D, Frank S, Probst A et al (2009) Transmission and spreading of tauopathy in transgenic mouse brain. Nat Cell Biol 11:909–913. https://doi.org/10.1038/ncb1901
Copenhaver BR, Rabin LA, Saykin AJ, Roth RM, Wishart HA, Flashman LA et al (2006) The fornix and mammillary bodies in older adults with Alzheimer’s disease, mild cognitive impairment, and cognitive complaints: a volumetric MRI study. Psychiatry Res Neuroimaging 147:93–103. https://doi.org/10.1016/j.pscychresns.2006.01.015
Crary JF, Trojanowski JQ, Schneider JA, Abisambra JF, Abner EL, Alafuzoff I et al (2014) Primary age-related tauopathy (PART): a common pathology associated with human aging. Acta Neuropathol 128:755–766. https://doi.org/10.1007/s00401-014-1349-0
Delaère P, Duyckaerts C, He Y, Piette F, Hauw JJ (1991) Subtypes and differential laminar distributions of beta A4 deposits in Alzheimer’s disease: relationship with the intellectual status of 26 cases. Acta Neuropathol 81:328–335. https://doi.org/10.1007/bf00305876
Duyckaerts C, Godefroy G (2000) Voronoi tessellation to study the numerical density and the spatial distribution of neurones. J Chem Neuroanat 20:83–92. https://doi.org/10.1016/s0891-0618(00)00064-8
Duyckaerts C, Hauw J-J (1997) Prevalence, incidence and duration of Braak’s stages in the general population: can we know? Neurobiol Aging 18:362–369
Duyckaerts C, Godefroy G, Hauw JJ (1994) Evaluation of neuronal numerical density by Dirichlet tessellation. J Neurosci Methods 51:47–69. https://doi.org/10.1016/0165-0270(94)90025-6
Duyckaerts C, Uchihara T, Seilhean D, He Y, Hauw J-J (1997) Dissociation of Alzheimer type pathology in a disconnected piece of cortex. Acta Neuropathol 93:501–507
Duyckaerts C, Braak H, Brion J-P, Buée L, Del Tredici K, Goedert M et al (2015) PART is part of Alzheimer disease. Acta Neuropathol 129:749–756. https://doi.org/10.1007/s00401-015-1390-7
Duyckaerts C, Sazdovitch V, Ando K, Seilhean D, Privat N, Yilmaz Z et al (2018) Neuropathology of iatrogenic Creutzfeldt-Jakob disease and immunoassay of French cadaver-sourced growth hormone batches suggest possible transmission of tauopathy and long incubation periods for the transmission of Aβ pathology. Acta Neuropathol 135:201–212. https://doi.org/10.1007/s00401-017-1791-x
Eisele YS, Duyckaerts C (2016) Propagation of Aβ pathology: hypotheses, discoveries, and yet unresolved questions from experimental and human brain studies. Acta Neuropathol 131:5–25
Eisele YS, Obermuller U, Heilbronner G, Baumann F, Kaeser SA, Wolburg H et al (2010) Peripherally applied Aβ-containing inoculates induce cerebral β-amyloidosis. Science 330:980–982. https://doi.org/10.1126/science.1194516
Falcon B, Zhang W, Murzin AG, Murshudov G, Garringer HJ, Vidal R et al (2018) Structures of filaments from Pick’s disease reveal a novel tau protein fold. Nature 561:137–140. https://doi.org/10.1038/s41586-018-0454-y
Falcon B, Zhang W, Schweighauser M, Murzin AG, Vidal R, Garringer HJ et al (2018) Tau filaments from multiple cases of sporadic and inherited Alzheimer’s disease adopt a common fold. Acta Neuropathol 136:699–708. https://doi.org/10.1007/s00401-018-1914-z
Falcon B, Zivanov J, Zhang W, Murzin AG, Garringer HJ, Vidal R et al (2019) Novel tau filament fold in chronic traumatic encephalopathy encloses hydrophobic molecules. Nature 568:420–423. https://doi.org/10.1038/s41586-019-1026-5
Fitzpatrick AWP, Falcon B, He S, Murzin AG, Murshudov G, Garringer HJ et al (2017) Cryo-EM structures of tau filaments from Alzheimer’s disease. Nature 547:185–190. https://doi.org/10.1038/nature23002
Furman JL, Holmes BB, Diamond MI (2015) Sensitive detection of proteopathic seeding activity with FRET flow cytometry. J Vis Exp. https://doi.org/10.3791/53205
Gallyas F (1971) Silver staining of Alzheimer’s neurofibrillary changes by means of physical development. Acta Morphol Acad Sci Hung 19:1–8
Gomes LA, Hipp SA, Rijal Upadhaya A, Balakrishnan K, Ospitalieri S, Koper MJ et al (2019) Aβ-induced acceleration of Alzheimer-related τ-pathology spreading and its association with prion protein. Acta Neuropathol 138:913–941. https://doi.org/10.1007/s00401-019-02053-5
Grignon Y, Duyckaerts C, Bennecib M, Hauw J-J (1998) Cytoarchitectonic alterations in the supramarginal gyrus of late onset Alzheimer’ s disease. Acta Neuropathol 95:395–406
Grundke-Iqbal I, Iqbal K, Tung YC, Quinlan M, Wisniewski HM, Binder LI (1986) Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc Natl Acad Sci 83:4913–4917. https://doi.org/10.1073/pnas.83.13.4913
Gundersen HJ, Osterby R (1981) Optimizing sampling efficiency of stereological studies in biology: or “do more less well!”. J Microsc 121:65–73
Guo JL, Narasimhan S, Changolkar L, He Z, Stieber A, Zhang B et al (2016) Unique pathological tau conformers from Alzheimer’s brains transmit tau pathology in nontransgenic mice. J Exp Med 213:2635–2654. https://doi.org/10.1084/jem.20160833
Hardy JA, Higgins GA (1992) Alzheimer’s disease: the amyloid cascade hypothesis. Science 256:184–185
Hauw J, Feske S, Amarenco P, De Girolami U (2019) Vascular pathology. In: Gray F, Duyckaerts C, De Girolami U (eds) Manual of basic neuropathology, 6th edn. Oxford University Press, Oxford, pp 82–121
He Z, Guo JL, McBride JD, Narasimhan S, Kim H, Changolkar L et al (2017) Amyloid-β plaques enhance Alzheimer’s brain tau-seeded pathologies by facilitating neuritic plaque tau aggregation. Nat Med 24:29–38. https://doi.org/10.1038/nm.4443
Héraud C, Goufak D, Ando K, Leroy K, Suain V, Yilmaz Z et al (2014) Increased misfolding and truncation of tau in APP/PS1/tau transgenic mice compared to mutant tau mice. Neurobiol Dis 62:100–112. https://doi.org/10.1016/j.nbd.2013.09.010
Hervé D, Porché M, Cabrejo L, Guidoux C, Tournier-Lasserve E, Nicolas G et al (2018) Fatal Aβ cerebral amyloid angiopathy 4 decades after a dural graft at the age of 2 years. Acta Neuropathol 135:801–803. https://doi.org/10.1007/s00401-018-1828-9
Holmes BB, Furman JL, Mahan TE, Yamasaki TR, Mirbaha H, Eades WC et al (2014) Proteopathic tau seeding predicts tauopathy in vivo. Proc Natl Acad Sci USA 111(41):E4376–E4385. https://doi.org/10.1073/pnas.1411649111
Hu W, Zhang X, Tung YC, Xie S, Liu F, Iqbal K (2016) Hyperphosphorylation determines both the spread and the morphology of tau pathology. Alzheimer’s Dement 12:1066–1077. https://doi.org/10.1016/j.jalz.2016.01.014
Iba M, McBride JD, Guo JL, Zhang B, Trojanowski JQ, Lee VM-Y (2015) Tau pathology spread in PS19 tau transgenic mice following locus coeruleus (LC) injections of synthetic tau fibrils is determined by the LC’s afferent and efferent connections. Acta Neuropathol 130:349–362. https://doi.org/10.1007/s00401-015-1458-4
Jaunmuktane Z, Mead S, Ellis M, Wadsworth JDF, Nicoll AJ, Kenny J et al (2015) Evidence for human transmission of amyloid-β pathology and cerebral amyloid angiopathy. Nature 525:247–250. https://doi.org/10.1038/nature15369
Jaunmuktane Z, Quaegebeur A, Taipa R, Viana-Baptista M, Barbosa R, Koriath C et al (2018) Evidence of amyloid-β cerebral amyloid angiopathy transmission through neurosurgery. Acta Neuropathol 135:671–679. https://doi.org/10.1007/s00401-018-1822-2
Kovacs GG, Lutz MI, Ricken G, Ströbel T, Höftberger R, Preusser M et al (2016) Dura mater is a potential source of Aβ seeds. Acta Neuropathol 131:911–923. https://doi.org/10.1007/s00401-016-1565-x
Lewis J, Dickson DW, Lin W, Chisholm L, Corral A, Jones G et al (2001) Enhanced neurofibrillary degeneration in transgenic mice expressing mutant Tau and APP. Science 293:1487–1491
Mann DMA, Hardy J (2013) Amyloid or tau: the chicken or the egg? Acta Neuropathol 126:609–613. https://doi.org/10.1007/s00401-013-1162-1
McKeith IG, Boeve BF, Dickson DW, Halliday G, Taylor J-P, Weintraub D et al (2017) Diagnosis and management of dementia with Lewy bodies. Neurology 89:88–100. https://doi.org/10.1212/WNL.0000000000004058
Meyer-Luehmann M, Coomaraswamy J, Bolmont T, Kaeser S, Schaefer C, Kilger E et al (2006) Exogenous induction of cerebral β-amyloidogenesis is governed by agent and host. Science 313:1781–1784. https://doi.org/10.1126/science.1131864
Mirbaha H, Chen D, Morazova OA, Ruff KM, Sharma AM, Liu X et al (2018) Inert and seed-competent tau monomers suggest structural origins of aggregation. Elife 7:e36584. https://doi.org/10.7554/eLife.36584
Plowey ED, Ziskin JL (2016) Hippocampal phospho-tau/MAPT neuropathology in the fornix in Alzheimer disease: an immunohistochemical autopsy study. Acta Neuropathol Commun 4:114. https://doi.org/10.1186/s40478-016-0388-2
Pooler AM, Polydoro M, Maury EA, Nicholls SB, Reddy SM, Wegmann S et al (2015) Amyloid accelerates tau propagation and toxicity in a model of early Alzheimer’s disease. Acta Neuropathol Commun 3:14. https://doi.org/10.1186/s40478-015-0199-x
Ritchie DL, Adlard P, Peden AH, Lowrie S, Le Grice M, Burns K et al (2017) Amyloid-β accumulation in the CNS in human growth hormone recipients in the UK. Acta Neuropathol 134:221–240. https://doi.org/10.1007/s00401-017-1703-0
Saito T, Mihira N, Matsuba Y, Sasaguri H, Hashimoto S, Narasimhan S et al (2019) Humanization of the entire murine Mapt gene provides a murine model of pathological human tau propagation. J Biol Chem 294:12754–12765. https://doi.org/10.1074/jbc.RA119.009487
Schmidt ML, Lee VM, Trojanowski JQ (1991) Comparative epitope analysis of neuronal cytoskeletal proteins in Alzheimer’s disease senile plaque neurites and neuropil threads. Lab Invest 64:352–357
Selkoe DJ, Hardy J (2016) The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol Med 8:595–608. https://doi.org/10.15252/emmm.201606210
Sharma AM, Thomas TL, Woodard DR, Kashmer OM, Diamond MI (2018) Tau monomer encodes strains. Elife. https://doi.org/10.7554/eLife.37813
Snedecor G, Cochran W (1980) Statistical methods, 7th edn. Iowa State University Press, Ames, pp 290–291
Takeda S, Wegmann S, Cho H, DeVos SL, Commins C, Roe AD et al (2015) Neuronal uptake and propagation of a rare phosphorylated high-molecular-weight tau derived from Alzheimer’s disease brain. Nat Commun 6:8490. https://doi.org/10.1038/ncomms9490
Tardivel M, Bégard S, Bousset L, Dujardin S, Coens A, Melki R et al (2016) Tunneling nanotube (TNT)-mediated neuron-to neuron transfer of pathological Tau protein assemblies. Acta Neuropathol Commun 4:117. https://doi.org/10.1186/s40478-016-0386-4
Thal DR, Rüb U, Orantes M, Braak H (2002) Phases of Aβ-deposition in the human brain and its relevance for the development of AD. Neurology 58:1791–1800
Thierry M, Marty S, Boluda S, Duyckaerts C (2017) Alzheimer’s senile plaque as shown by microcryodissection, a new technique for dissociating tissue structures. J Neural Transm 124:685–694. https://doi.org/10.1007/s00702-017-1718-7
Uchihara T, Kondo H, Ikeda K, Kosaka K (1995) Alzheimer-type pathology in melanin-bleached sections of substantia nigra. J Neurol 242:485–489
Vann SD, Aggleton JP (2004) The mammillary bodies: two memory systems in one? Nat Rev Neurosci 5:35–44. https://doi.org/10.1038/nrn1299
Vergara C, Houben S, Suain V, Yilmaz Z, De Decker R, Vanden Dries V et al (2019) Amyloid-β pathology enhances pathological fibrillary tau seeding induced by Alzheimer PHF in vivo. Acta Neuropathol 137:397–412. https://doi.org/10.1007/s00401-018-1953-5
von Bergen M, Friedhoff P, Biernat J, Heberle J, Mandelkow E-M, Mandelkow E (2000) Assembly of tau protein into Alzheimer paired helical filaments depends on a local sequence motif (306VQIVYK311) forming beta structure. Proc Natl Acad Sci 97:5129–5134. https://doi.org/10.1073/pnas.97.10.5129
Wang Y, Balaji V, Kaniyappan S, Krüger L, Irsen S, Tepper K et al (2017) The release and trans-synaptic transmission of Tau via exosomes. Mol Neurodegener 12:5. https://doi.org/10.1186/s13024-016-0143-y
Weibel ER (1979) Stereological methods, vol. 1: practical methods for biological morphometry. Academic, London
Acknowledgements
This project received financial support from France Alzheimer (project “Propagation of Tau and Aβ aggregation through axons in the subiculo-fornico-mamillary system: tracking propagons” and Ibisa (Project “Neuro-CEB better”). Manon Thierry was funded by a doctoral scholarship from the Ministry of Research and Higher Education, attributed by the doctoral school “Physiology, Physiopathology and Therapeutics-ED394” (Sorbonne University). The human samples were obtained from the Neuro-CEB brain bank (BRIF Number 0033-00011), partly funded by the patients’ associations ARSEP, ARSLA, “Connaître les Syndromes Cérébelleux”, France-DFT, France Parkinson and by Vaincre Alzheimer Fondation, to which we express our gratitude. We are also grateful to the patients and their families. We thank Sabrina Leclère-Turbant and Marie-Claire Artaud for their technical and managerial assistance with post-mortem human samples. The brain bank is hosted by the Biological Resources Platform of Pitié-Salpêtrière Hospital, APHP. Brain clarification with CLARITY was performed on the ICM HISTOMICS platform. Cell culture experiments were performed on the ICM CELIS platform. Biphoton, confocal and electronic microscopy pictures were acquired on the ICM.QUANT imaging platform. We thank the technical team of the Raymond Escourolle neuropathology department as well as the technical staff involved at ICM, including Valérie Thuries and Annick Prigent for their help and technical advices in histology, Julien Dumont for his assistance and expertise in CLARITY-cleared tissue imaging and analysis and Dominique Langui and Asha Baskaran for their help and knowledge in electron microscopy analysis. We also thank Jean-Pierre Brion and Kunie Ando for their valuable advices and discussion. The Neuro-CEB Neuropathology network includes: Dr. Franck Letournel (CHU Angers), Dr. Marie-Laure Martin-Négrier (CHU Bordeaux), Pr. Françoise Chapon (CHU Caen), Pr. Catherine Godfraind (CHU Clermont-Ferrand), Pr. Claude-Alain Maurage (CHU Lille), Dr. Vincent Deramecourt (CHU Lille), Dr. David Meyronnet (CHU Lyon), Dr. Nathalie Streichenberger (CHU Lyon), Dr. André Maues de Paula (CHU Marseille), Pr. Valérie Rigau (CHU Montpellier), Dr. Fanny Vandenbos-Burel (Nice), Pr. Charles Duyckaerts (CHU PS Paris), Pr. Danielle Seilhean (CHU PS, Paris), Dr. Susana Boluda (CHU PS, Paris), Dr. Isabelle Plu (CHU PS, Paris), Dr. Serge Milin (CHU Poitiers), Dr. Dan Christian Chiforeanu (CHU Rennes), Pr. Annie Laquerrière (CHU Rouen), Dr. Béatrice Lannes (CHU Strasbourg).
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The members of Brainbank Neuro-CEB Neuropathology Network are listed in the Acknowledgements section.
Electronic supplementary material
Below is the link to the electronic supplementary material.
401_2019_2108_MOESM1_ESM.avi
Supplementary material 1 (AVI 35884 kb) Video 1 (online resource) CLARITY in the mamillary body showing Aβ diffuse deposit surrounded by Tau positive neurites in three dimensions. Human brain, anti-Aβ 4G8/Tau B19 immunohistochemistry, Aβ in green (Alexa 488), Tau in red (Alexa 555)
401_2019_2108_MOESM2_ESM.avi
Supplementary material 2 (AVI 31367 kb) Video 2 (online resource) CLARITY in the mamillary body. Aβ neuritic deposit surrounded by Tau positive neurites in three dimensions. Human brain, anti-Aβ 4G8/Tau B19 immunohistochemistry, Aβ in green (Alexa 488), Tau in red (Alexa 555)
401_2019_2108_MOESM3_ESM.avi
Supplementary material 3 (AVI 137117 kb) Video 3 (online resource) General view of the fornix ending in the mamillary body where Tau and Aβ pathologies converged. Three-dimensional view after CLARITY. Human brain, anti-Aβ 4G8/Tau B19 immunohistochemistry, Aβ in green (Alexa 488), Tau in red (Alexa 555)
401_2019_2108_MOESM5_ESM.tif
Supplementary material 5 (TIFF 43880 kb) Supplementary Fig. 1 (online resource) Aβ volume densities in the subiculo-fornico-mamillary system. Each case is represented by a dot (values obtained in the subiculum), square (values obtained in the pillar of the fornix) and triangle (values obtained in the mamillary body). The volume densities of Aβ deposits (or amyloid loads) are represented as a function of Braak stages (Kruskal–Wallis and Dunn’s post tests in comparison with the control group, which included cases at Braak stages 0/I/II, *** p < 0.001, **** p < 0.0001). Error bars: standard error of the mean
401_2019_2108_MOESM6_ESM.tif
Supplementary material 6 (TIFF 214293 kb) Supplementary Fig. 2 (online resource) Relationship between Tau pathology in the various regions of the subiculo-fornico-mamillary system. a “Observed”: the severity of Tau lesions was graded 0 to 3. The three contingency tables show the number of cases corresponding to the observed association of grades between the two areas represented on the X and Y axes. b “Observed–Expected”. “Expected” means expected number in the case of a random distribution: it is the product of the total number of cases in the corresponding row, times the total number in the corresponding column divided by the total number of cases (as in a Chi²). The difference between the “Observed” and “Expected” numbers of cases is shown in Table b. A positive difference indicates an excess of the observed number of cases, in comparison with a random distribution and conversely. The cells containing too many observed cases (by comparison with the expected number) are coloured in red tones and the ones containing too few in green tones. The distribution of the enriched cells (coloured in red) located along a diagonal or parallel to it in all tables, suggests correlations of Tau pathology severities between the various regions of the subiculo-fornico-mamillary system. c Linear regressions of Tau lesion severities, between each region of the subiculo-fornico-mamillary system. The computation is made under the hypothesis that the semi-quantitative scores of Tau pathology approximately describe a continuous gradient of severity: under this hypothesis, intermediate values may be interpolated by the regression line. Pearson’s r and p-value are indicated in red. The diameter of the dots is proportional to the number of corresponding cases (as indicated in the figure). The equations of the regression lines are: Tau grade in the pillar of the fornix = 0.9921 × Tau grade in the subiculum + 0.2661. Tau grade in the mamillary body = 0.5241 × Tau grade in the pillar of the fornix − 0.1144. Tau grade in the mamillary body = 0.5499 × Tau grade in the subiculum − 0.0109
401_2019_2108_MOESM7_ESM.tif
Supplementary material 7 (TIFF 144922 kb) Supplementary Fig. 3 (online resource) Scatterplots showing the linear regressions of the volume densities of Aβ pathology (Y-axis) as a function of the semi-quantitative scores of Tau pathology (X-axis) between each region of the subiculo-fornico-mamillary system. As in Supplementary Fig. 2, we postulated that the semi-quantitative scores of Tau pathology approximated a continuous gradient of severity and that the intermediate values were interpolated by the regression line. Pearson’s r and p-value are indicated in red
Rights and permissions
About this article
Cite this article
Thierry, M., Boluda, S., Delatour, B. et al. Human subiculo-fornico-mamillary system in Alzheimer’s disease: Tau seeding by the pillar of the fornix. Acta Neuropathol 139, 443–461 (2020). https://doi.org/10.1007/s00401-019-02108-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-019-02108-7