Abstract
Key message
A plant-based multiepitopic protein (LTBentero) containing epitopes from ETEC, S. typhimurium, and V. parahaemolyticus was produced in plants cells and triggered systemic and intestinal humoral responses in immunized mice.
Abstract
Around 200 million people suffer gastroenteritis daily and more than 2 million people die annually in developing countries due to such pathologies. Vaccination is an alternative to control this global health issue, however new low-cost vaccines are needed to ensure proper vaccine coverage. In this context, plants are attractive hosts for the synthesis and delivery of subunit vaccines. Therefore, in this study a plant-made multiepitopic protein named LTBentero containing epitopes from antigens of enterotoxigenic E. coli, S. typhimurium, and V. parahaemolyticus was produced and found immunogenic in mice. The LTBentero protein was expressed in tobacco plants at up to 5.29 µg g−1 fresh leaf tissue and was deemed immunogenic when administered to BALB/c mice either orally or subcutaneously. The plant-made LTBentero antigen induced specific IgG (systemic) and IgA (mucosal) responses against LTB, ST, and LptD epitopes. In conclusion, multiepitopic LTBentero was functionally produced in plant cells, being capable to trigger systemic and intestinal humoral responses and thus it constitutes a promising oral immunogen candidate in the fight against enteric diseases.
Similar content being viewed by others
Introduction
Enteric diseases remain as a remarkable worldwide public health problem. Around 200 million people suffer gastroenteritis daily and more than 2 million people die annually due to such infections in developing countries, mainly infants (WHO 2010). Prominent pathogens causing enteric diseases include adenovirus; astro-virus; rotavirus; Campylobacteria; Shigella; Salmonella; E. coli; and Vibrios, particularly V. cholerae (Girard et al. 2006). Enteric pathogens are transmitted as a consequence of inadequate sanitation in both water and food, conditions that will prevail in developing countries (WHO 2017). Enterotoxigenic E. coli (ETEC) is the most common bacterium-causing diarrhea (Walker et al. 2007). Annually, ETEC affects around 400 million people and is responsible for 300,000–500,000 deaths (Zheng et al. 2005). V. cholerae is responsible for 3–5 million of infections and about 100,000–130,000 deaths per year (WHO 2010). Salmonella persists as a major public health threat related to the consumption of poultry in developed countries (Majowicz et al. 2010). The Center for Disease Control and Prevention (2008) estimates that Salmonella causes 1.4 million of infections and about 600 deaths each year in the United States. In Asian countries such as Japan, V. parahaemolyticus is associated with 30% of food-related poisonings (Broberg et al. 2011) due to the high consumption of undercooked fish and shellfish (Datta et al. 2008); this pathogen is also considered one of the biggest economic problems in aquaculture (Liu et al. 2011a, b).
Enteric diseases caused by bacteria are typically treated with antibiotics, however their inadequate use has generated resistant strains and thus prophylactic approaches are the ideal goal to reduce their impact (Gordon et al. 2008). Vaccination is a viable alternative to prevent enteric infections and thus decrease the associated morbidity and mortality. To achieve this goal, developing low cost oral vaccines is critical in view to the budget limitations that often reduce vaccination coverage (Walker et al. 2007). In fact, oral immunization for enteric diseases is highly convenient since it leads to humoral responses in the gastrointestinal tract, which constitutes the site of entry of enteric pathogens; thus vaccines based in this approach are viable.
Plant-based vaccines constitute an alternative for oral immunization at low costs (Takeyama et al. 2015). The use of the plant cell for synthesis and delivery of functional antigens is a well-established technology; offering several advantages such as low cost, easy scalability, absence of human pathogens replication, and proper synthesis of complex heterologous proteins (Scotti and Rybicki 2013; Rosales-Mendoza et al. 2016). Thus far several antigens from bacterial pathogens, including toxin subunits, have been expressed at sufficient levels leading to promising vaccination models (Rosales-Mendoza et al. 2009; Koya et al. 2005).
One of the challenges on vaccine development is the fact that there are infections caused by concomitant serotypes, strains or species (Lun et al. 2014; Wang et al. 2013), thus polyvalent vaccines are required (Peng et al. 2016). New computer and molecular technologies allow the generation of multiepitopic recombinant vaccines capable of triggering immunity against several pathogens using a single antigen (Ruan et al. 2015). Another challenge in this field is the poor immunogenic activity that is often observed for subunit vaccines, thus requiring adjuvants to induce proper immune responses in terms of potency and type (Chauhan et al. 2017). Several proteins have been applied for this purpose, including the B subunits of cholera toxin (CTB) or the heat labile enterotoxin (LTB) from ETEC, which are potent mucosal adjuvants (Adkins et al. 2012; Al-Barwani et al. 2014). The immunogenic characteristics of LTB and CTB result in part from their ability to bind the GM1 receptor that facilitates the antigen reaching the submucosa, and favors uptake by dendritic cell as well as B and T cells effector functions (Yamamoto et al. 2001).
In this study, a plant-based immunogen against enteric diseases was developed, based on a chimeric protein (LTBentero) comprising LTB as adjuvant/carrier and epitopes from ETEC, S. typhimurium, and V. parahaemolyticus. Tobacco plants carrying the ltbentero gene-coding gene were developed, and protein yields and the immunogenic activity in mice were determined.
Materials and methods
Design of multiepitopic genes and molecular cloning
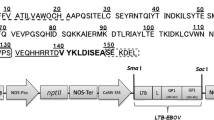
The multiepitopic gene was designed based on epitopes from known antigenic determinants of the following enteric pathogens: ST (SNSSNYCCELCCNPACTGCYV) from ETEC, FliC (VQNRFNSAITNLGNT) from S. typhimurium and LptD (WENQAIGSTGSSPEY) from V. parahaemolyticus (Jacob et al. 1983, 1985; Newton et al. 1989; Bergman et al. 2005; Kremer et al. 2011; Rosales-Mendoza et al. 2011; Zha et al. 2016; Table 1). In addition, the B subunit of the LT toxin produced by ETEC was included as an immunogenic carrier. To avoid undesired junctional epitopes and to provide appropriate folding/display for immune recognition, a proline-containing linker (GPGP) was incorporated between the LTB sequence and the target epitopes (Livingston et al. 2002). The signal peptide sequence of the vegetative storage protein from Glycine max was incorporated at the N-terminus of the fusion protein whereas an endoplasmic reticulum retention sequence (SEKDEL) was included at the C-terminus. The gene sequence was flanked with the SacI and SmaI restriction sites to facilitate subcloning into the pBI121 binary vector downstream of the CaMV 35S promoter (Fig. 1). The LTBentero coding gene was codon-optimized for plants and synthesized by GenScript® (Piscataway, NJ). Subcloning was performed under conventional procedures to yield the construct named pBI121LTBentero. The recombinant plasmid obtained in E. coli Top 10 was identified by restriction analysis with the enzyme HindIII and then transferred into Agrobacterium tumefaciens (GV3101 strain) by electroporation. A positive A. tumefaciens clone was propagated and used for tobacco transformation experiments.
Map of the expression vector used to produce the LTBentero multiepitopic protein in plants. The LTBentero gene comprises the signal peptide from the Glycine max vegetative storage protein, the full-length mature sequence of the E. coli heat-labile enterotoxin B subunit, a four aminoacid linker, followed by the epitopes from antigens ST, FliC and LptD and the SEKDEL endoplasmic reticulum (E.R) retention signal. The LTBentero-coding gene was cloned into the pBI121 vector to drive its expression under the constitutive 35SCaMV promoter. The vector possesses the nptII as gene marker that confers kanamycin resistance in the transformed plants
Tobacco transformation
Transgenic tobacco plants (Nicotiana tabacum cv. Petite Havana SR1) were obtained following the method described by Horsch et al. (1985). Briefly, tobacco leaf sections (5 mm × 5 mm) from in vitro-obtained plantlets were inoculated with an overnight-grown culture of recombinant Agrobacterium (OD600nm = 0.5), co-cultured onto RMOP medium (MS basal medium supplemented with 3% sucrose, 1 mg L−1 benzyladenine, 0.1 mg L−1 naphthaleneacetic acid, 1 mg L−1 thiamine, and solidified with 3% agar; pH 5.8) at 28 °C for 48 h in darkness. Thereafter, explants were cultured under selective pressure until shoot development (RMOP medium supplemented with 250 mg L−1 cefotaxime and 100 mg L−1 kanamycin). Shoots were subsequently rooted in MS medium lacking plant growth regulators. Regenerated plants were transferred to soil and grown in a greenhouse under a 16-h photoperiod with a light intensity of 100 µmol m−2 s−1 and 30% of relative humidity. Leaf tissues were collected from mature plants and freeze-dried in a LABCONCO system (FreeZone 6 L) at − 50 °C (collector temperature) for 48 h. Dry material was subsequently milled and stored at room temperature until further use.
Transgene detection by PCR
Detection of ltbentero transgene was performed by PCR analysis using the following primers: forward 5′CGCACAATCCCACTATCCTTCGC 3′, targeting the 35S promoter; and reverse 5′AGGGTTTCGCTCATGTGTTGAGC 3′, targeting the terminator NOS. Total DNA preparations were obtained from putative transformants and wild-type (WT) plants according to the method described by Dellaporta et al. (1983). 25 µL-reaction PCR mixtures contained the following components: 100 ng of test DNA, 1 × PCR buffer, 1.5 mM magnesium chloride, 2.5 U Taq DNA polymerase (Vivantis), 1 mM dNTPs, and 1 ∝ M of each forward and reverse specific primers, which are designed to yield a 769 bp amplicon. Temperature cycling conditions were: 94 °C for 5 min (initial denaturation); 35 cycles comprising the following steps: 94 °C for 30 s (denaturation), 56 °C for 60 s (annealing), and 72 °C for 60 s (elongation); and a final extension at 72 °C for 5 min. Thermal cycling was performed in a MultiGeneTM Mini Personal Thermal Cycler (Labnet). The presence of ltbentero amplicons was determined by electrophoresis in 1% agarose gels.
Detection of plant-made LTBentero
For detection and quantification of the LTBentero recombinant protein, hyperimmune sera (polyclonal antibodies) were generated in mice using pure recombinant LTB and synthetic ST (GenScript®, Piscataway, NJ), following a previously reported protocol (Ríos-Huerta et al. 2017). Briefly, 10-week old male BALB/c mice were immunized on day 1 in the rear footpad with 10 µg of the ST or LTB emulsified with 10 µL of complete Freund’s adjuvant. The subsequent doses were intraperitoneally administered on days 8, 15, 22, 29, and 36; consisting of 50 µg of ST or LTB emulsified in one volume of incomplete Freund’s adjuvant. Mice were bled at day 43 to measure antibody titers. The animals were subsequently sacrificed to collect sera. Animal handling was conducted according to the Guide for Care and Use of Laboratory Animals of the National Institute of Health (USA) and protocols approved by the Committee on Research Ethics of the Faculty of Chemistry/University of San Luis Potosi (Permit Number: CEID-2013-004).
To detect the presence and integrity of the recombinant protein produced by the transformed plants, Dot and Western blot analyses were performed. Fresh leaf tissue from transgenic or WT plants (100 mg) were milled in the presence of 300 µL of protein extraction buffer (750 mM Tris–HCl pH 8.0 15% Sucrose, 1 mM proteinase inhibitor PMSF). For Western blot analysis, soluble proteins were mixed with 1 × reducing loading buffer (50 mM Tris–HCl pH 6.8, 100 mM DTT, 2% SDS 0.1% bromophenol blue, 10% glycerol). The samples were denatured by boiling for 5 min at 95 °C. Protein extracts were resolved by SDS-PAGE using 4–12% acrylamide gels under denaturing conditions. The gel was blotted onto a BioTrace PVDF membrane (Pall Corporation, NY) using a TV100-EBK Electroblotter (AlphaMetrix Biotech, GER) for 1 h at 150 V in a methanol-based transfer buffer. After blocking (incubation in 5% fat-free dry milk for 5 h at 25 °C), blots were incubated overnight at 4 °C with the anti-LTB serum (1:500). Pure recombinant LTB was included in the analysis as positive control. Immunoreactivity was detected by labeling with a horseradish peroxidase-conjugated goat anti-mouse antibody (1:2000; Sigma, St. Louis, MO) and a subsequent 2 h-incubation at room temperature with the SuperSignal West Dura solution following the instructions from the manufacturer (Thermo Scientific). For Dot blot analysis protein samples were directly applied onto a BioTrace PVDF membrane (Pall Corporation, NY) and synthetic ST was used as a positive control. After blocking (incubation in 5% fat-free dry milk for 5 h at 25 °C), blots were incubated overnight at 4 °C with the anti-ST serum (1:200). Inmunoreactivity was detected as mentioned above in the Western blot Analysis.
ELISA was performed to quantify LTBentero levels in plant leaf tissues of the six different lines (P2, P4, P5, P6, P8 and P9), using tissues from a WT plant as a negative control. Protein extracts were obtained as described above. Extracts were clarified by centrifugation (16,000×g at 4 °C for 15 min), and the supernatants were diluted 1:16 in carbonate buffer (15 mM Na2CO3, 35 mM NaHCO3) and used to coat ELISA plates (4 °C, overnight incubation). Three washes with PBST were conducted between each incubation step. The plates were blocked with 5% fat-free dry milk dissolved in PBS for 2 h at room temperature. Thereafter, anti-LTB serum produced as mentioned above (diluted 1:500) was used for primary labeling (4 °C, overnight incubation). Secondary labeling was performed with a goat anti-mouse horseradish peroxidase-conjugated antibody diluted 1:2000 (Sigma, St. Louis, MO; incubation at 25 °C for 2 h). The final step comprised a 30 min-incubation with the substrate solution containing 0.3 mg mL−1 2,2-Azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS; Sigma, St. Louis, MO) and 0.1 mM H2O2. OD values were recorded at 405 nm using an iMark™ microplate reader (Bio-Rad, Hercules, CA). Pure recombinant LTB was used to construct a calibration curve to quantify LTBentero levels in tobacco leaf tissues (expressed as µg per gram of fresh leaf tissue, µg g−1 FW). The background signal from the negative control (extract from WT plant) was subtracted from the OD values from the test extracts.
Immunogenicity assessment
Immunogenicity of the LTBentero chimeric protein was evaluated in 12-week old female BALB/c mice. Test mice groups (n = 4) were subjected to oral (p.o.) or subcutaneous (s.c.) immunization with tobacco leaf material from transgenic line P2 (LTBentero group) or the WT line (WT group). For subcutaneous immunization doses were prepared by milling 10 mg of fresh leaf tobacco tissues in 300 µL of PBS, and subsequently clarified by centrifugation; injected supernatants contained ≈ 70 ng of LTBentero. For oral immunizations 50 mg of tobacco tissues (containing ≈ 300 ng of LTBentero) were milled in the presence of 300 µL of PBS and the crude extract was administered intragastrically. The immunization scheme comprised 4 weekly immunizations (on days 1, 8, 15, and 22). At day 29, sera and feces samples were obtained and stored at − 80 °C until antibody content analysis.
The levels of anti-LTBentero antibodies in serum and feces samples were measured by ELISA. Plates were coated overnight at 4 °C with LTB (250 ng per well), or ST, LptD, and FliC synthetic peptides (1 µg per well) and subsequently blocked with 5% fat free dry milk for 2 h at 25 °C. Plates were incubated overnight at 4 °C with serial dilutions of sera prepared in PBS (1:20 to 1:160 dilutions for IgG determination and 1:20 for IgG subclasses). For feces analysis, samples were resuspended in PBST supplemented with 1 mM PMSF and 5% non-fat milk; and clarified by centrifugation at 16,000×g at 4 °C for 15 min and loaded directly into the wells. Secondary labeling was carried out with mouse horseradish peroxidase-conjugated anti-mouse antibodies against IgG for sera or IgA for feces (1:2000 dilution, Sigma) at 25 °C for 1 h. Immunodetection was revealed by incubation with the substrate (ABTS) plus 0.1 mM of H2O2 for 60 min. OD values at 405 nm were measured in a microplate reader. Titers at each time point for each experimental group were calculated as the reciprocal of the highest dilution of sera having a mean OD value above the OD value from the WT group plus 2 × SD.
Results
Design of multiepitopic gene and vector construction
The first aim of this work was to identify epitopes reported in previous works with high immunoprotective efficacy against the target enteric pathogens. Among them, LTB and ST antigens from ETEC, FliC from S. typhimurium, and LptD from V. parahaemolyticus have been reported as effective immunoprotective antigens. Based on the literature, the most relevant epitopes for each antigen were chosen to be included in the multiepitopic antigen (Table 1). The obtained synthetic gene (named ltbentero) was successfully cloned into the pBI121 vector (Fig. 1) as evidenced by the restriction profiles (data not shown).
Presence of ltbentero transgene in tobacco lines
Several transgenic lines were obtained from the kanamycin resistance screening after performing A. tumefaciens-mediated transformation. Tobacco transgenic plants were regenerated upon a period of 2 months after co-cultivation and twelve independent lines designated as P1–P12 were selected from individual explants for their characterization. Once the plants were acclimatized in soil under greenhouse conditions, the transgenic lines showed a normal and healthy development pattern (Fig. 2). DNA samples from each transgenic line were analyzed by PCR observing the presence of the ltbentero transgene (769 bp amplicon) in eight of twelve lines (Fig. 3a), whereas the WT sample showed no amplification.
LTBentero transgene and protein detection. a PCR analysis was conducted using genomic DNA extracted from putative transgenic or WT tobacco plants and primers landing at the 35S promoter and the NOS terminator. b Dot Blot analysis using leaf protein extracts from transgenic or WT plants using a mouse anti-ST serum to confirm LTBentero production. c Western blot analysis using a mouse anti-LTB serum to assess the presence of the LTBentero protein
Tobacco plants express the LTBentero recombinant protein
Dot blot analysis with anti-ST serum revealed positive reactivity in seven of the eight lines, whereas WT tobacco plant showed no signal (Fig. 3b). Anti-LTB Western blot analysis allowed the detection of a 28 kDa protein in six out of seven lines; whereas this protein band was not observed in the WT tobacco line (Fig. 3c). ELISA using anti-LTB serum for labeling was run to determine the accumulation levels of the LTBentero antigen in transgenic tobacco lines, revealing that six lines accumulated LTBentero at detectable levels ranging from 0.07 to 5.29 µg g−1 fresh leaves weight, with the line P2 having the highest expression levels (Fig. 4).
Accumulation levels of the LTBentero protein in transgenic tobacco plants determined by ELISA. Immunodetection was performed in leaf protein extracts from transgenic plants or WT plant as a negative control using a mouse anti-LTB serum to quantify LTBentero. A standard curve made with pure LTB was used to determine the levels of the recombinant protein in each line
Immunogenicity assay of plant-made LTBentero
Immunogenicity of the plant-made LTBentero protein was assessed in BALB/c mice, which were either subcutaneously or orally immunized with leaf tissue extracts from transgenic tobacco line P2. Significant anti-LTB (s.c. mean titer = 160 and p.o. mean titer = 80) and anti-ST (mean titer = 80) IgG levels were observed in mice sera after the second immunization in both subcutaneously and orally immunized mice (Fig. 5). The measurement of anti-LTB (mean titer = 8) and anti-ST (mean titer = 4) IgA levels in feces of subcutaneously immunized mice showed a significant response after the third immunization with respect to the control group (Fig. 6); whereas significant anti-LTB (mean titer = 4) and anti-ST (mean titer = 8) IgA levels were detected in feces of orally immunized after the fourth and third immunization, respectively (Fig. 6). In addition, significant anti-LptD (mean titer = 20) IgG levels were also observed in sera from subcutaneously and orally immunized mice after the third immunization (Fig. 5). Similarly, significant anti-LptD (mean titer = 8) IgA levels were detected in feces after the third oral immunization (Fig. 7). In contrast, no significant anti-FliC IgG systemic responses were detected in neither orally nor subcutaneously immunized mice (data not shown). However, significant anti-FliC (mean titer = 2) IgA levels were observed after the fourth oral immunization in the feces (Fig. 7).
Systemic IgG antibody response induced in mice by the LTBentero plant-made protein. a anti-LTB b anti-ST and c anti-LptD antibody titers in subcutaneously (s.c.) or orally (per os, p.o.) immunized mice. Antibody levels were determined by ELISA weekly over a period of 4 weeks post-first immunization.
Discussion
In the present study the design and expression in plants of a multiepitopic protein targeting several enteric pathogens was achieved as an approach to generate an attractive polyvalent oral vaccine candidate. This plant-based multiepitopic protein was designed based on immunoprotective epitopes from the following enteric pathogens: ETEC, V. parahaemolyticus, and S. typhimurium (Jacob et al. 1983, 1985; Newton et al. 1989; Rosales-Mendoza et al. 2011; Zha et al. 2016; Bergman et al. 2005; Kremer et al. 2011). LTB was chosen as the carrier of the multiepitopic arrangement for two reasons: (1) its immune-enhancing capacity in mucous membranes (Yamamoto et al. 2001), and (2) it is known to serve as immunmoprotective antigen against LT and CT toxins from ETEC and V. cholerae, respectively (Nashar et al. 2001). Although ST causes severe diarrhea, it is a small peptide that lacks of immunogenic activity itself. However, ST can become immunogenic when is coupled to other antigens, such as LTB (Girard et al. 2006; Klipstein et al. 1986; Pereira et al. 2001). In addition, an epitope from the S. typhimurium fliC flagellin was selected; this sequence is highly conserved in the genus Salmonella (Bergman et al. 2005). An epitope from the V. parahaemolyticus outer membrane protein (OMP) LptD was also incorporated; this protein is part of a large complex that is responsible for producing Lipopolysaccharide (LPS) in the cell membrane of these bacteria. On this regard, Zha et al. (2016) reported antibodies able to block LptD by binding to different sites and decreased LPS production with a subsequent bacterial clearance.
The production of the functional LTBentero multiepitopic protein in plants was explored in an effort to develop a low cost oral vaccine. The modified tobacco plants carrying the ltbentero gene were obtained and confirmed by PCR. Dot blot analysis allowed the detection of the protein whereas its expected molecular weight (28 kDa) was confirmed by Western blot analysis. According to ELISA data, LTBentero expression levels ranged 0.029–5.29 µg g−1 FW, which are in the range (24.5–2 µg g−1 ) of the observed yields for LTB or CTB chimeras produced in tobacco, lettuce, tomato, and Arabidopsis thaliana (Ríos-Huerta et al. 2017; Rosales-Mendoza et al. 2011; Martínez-González et al. 2011; Rigano et al. 2004; Walmsley et al. 2003). The difference in antigen yields among the transformed lines may be due to differential transgene insertion sites into the tobacco genome (Kim et al. 2007). Although there is an apparent lack of correlation between the expression levels determined by ELSIA and the band intensity observed in the Western blot analysis, it should be considered that ELISA is a more accurate technique in which several serial dilutions were analyzed, whereas in western blot a single dilution containing a fixed amount of total protein was analyzed and thus the detected signal could be masked by saturation effects (Bass et al. 2016). Therefore, the selection of the most productive line was based on ELISA data.
Interestingly no phenotypic alterations were observed in the tobacco lines, including those with the highest expression levels. This observation is notable since other bacterial toxin subunits have exerted toxicity in some plant species. For instance, Mason et al. (1998) reported that high levels of LTB in potato plants led to a severely stunted phenotype with slow shoot growth and poor tuber yield.
Immunogenicity evaluated by ELISA revealed that the plant-made LTBentero protein induced specific IgG (sera) and IgA (feces) against LTB, ST, and LptD epitopes in subcutaneously and orally immunized mice. Interestingly, high levels of systemic IgG antibodies were observed in both subcutaneously and orally immunized mice after the second immunization against LTB or ST and after third immunization for LptD. Regarding IgA mucosal production, high levels of antibodies against LTB and ST were detected after the third subcutaneous and oral immunization (except for anti-LTB in orally immunized mice). The immunogenic properties observed for the LTBentero chimeric protein suggests that it was correctly assembled and functionally produced in the plant cell. LTB is recognized as a mucosal adjuvant related with its efficient uptake by epithelial and dendritic cells, which favors the induction of adaptive immune responses in the submucosa (Lazorova et al. 1993). Moreover, the adjuvant activity of LTB fused with other peptides in plant-based vaccines has been demonstrated elsewhere (Rosales-Mendoza et al. 2009; Hongli et al. 2013). In line with our findings, significant specific IgG levels in serum and IgA levels in the large intestine were detected in mice after oral immunization with maize and carrot-made LTB (Chikwamba et al. 2002; Rosales-Mendoza et al. 2008). Oral immunogenicity of LTB encapsulated in plant cells has been confirmed in humans (Tacket et al. 1998).
The ST antigen cannot be used directly as a vaccine component due to its poor immunogenicity unless it is coupled to a carrier protein (Klipstein et al. 1986). Therefore, genetic fusions between ST and carrier proteins have been reported in an effort to successfully immunize against ST (Pereira et al. 2001). LTB fused with ST resulted in the induction of ST antibodies in test animals (Saarilahti et al. 1989; Clements 1990; Zheng et al. 2005; Zhang et al. 2010; Liu et al. 2011a, b; You et al. 2011). Interestingly, similar to our results, Deng et al. (2013) immunized mice (with 4 doses at 1-week intervals) with a ST peptide or a ST-LTB fusion protein using nanoparticles as carriers. The authors found a maximum production of specific IgG in serum and IgA in intestinal mucosa against both ST-LTB fusion protein and ST peptide at day 28. Interestingly, a tobacco plant-made LTB:ST protein induced significant anti-LTB IgG (serum) and IgA (feces) levels in orally immunized mice after a third immunization at similar levels to those elicited by the pure recombinant LTB (Rosales-Mendoza et al. 2009, 2011). In agreement with all these reports, our data also shows that mice immunized with LTBentero induced humoral responses against both LTB and ST at the systemic and intestinal levels.
Interestingly, this constitutes the first report on the immunogenicity of an LptD epitope encapsulated in plant cells. Li et al. (2014) reported LptD from V. parahaemolyticus as a highly immunogenic protein using an immunoproteomic approach. LptD is an integral OMP that along with other 6 proteins (LptA, B, C, E, F and G) constitute a trans-membrane complex responsible for transporting LPS (Freinkman et al. 2012; Xiang et al. 2014). LPS not only plays critical roles in protecting bacteria from harsh environments and in colonizing the host or evading attacks from the host immune system, but also forms a permeation barrier that prevents entering hydrophobic antibiotics to the microorganism, thus conferring antibiotic resistance (Ruiz et al. 2009; Li et al. 2015). Therefore, LptD is an attractive target for the development of vaccines and therapeutics. Moreover, Zha et al. (2016) showed that LptD is highly conserved and shared surface epitopes among pathogenic Vibrio species with the ability to induce immunoprotection against Vibrio infection in mice. Remarkably, sera from LptD-immunized mice reduced bacterial growth and LPS levels and increased the bacterial susceptibility to antibiotics. In contrast, in this study no significant IgG levels were found in serum against the FliC epitope. However, significant feces anti-FliC IgA levels were observed in mice subjected to four oral immunizations. It is proposed that the FliC is displayed in the context of the chimeric arrangement but optimizing the immunization protocol is required to increase the response against this target epitope. One alternative to be contemplated is combining the use of this plant-made chimeric antigen with boostings performed with a FliC antigen (e.g. a synthetic peptide). Salmonella expresses two types of flagellin (FljB and FliC) known as strong immunogens. FljB has been expressed in plants, being capable to induce humoral responses in subcutaneously immunized mice (Bergeron-Sandoval et al. 2011). The FliC protein flagellar subunit is an antigen capable to induce Th responses via macrophage-mediated presentation, stimulating antibody production; and it has become a candidate for vaccine development because it possesses a broad protective activity against multiple salmonella species (Cummings et al. 2006; Cookson and Beavan 1997; McSorley et al. 2000).
The positive results in terms of immunoreactivity and immunogenicity observed for the plant-made LTBentero merit further evaluations in challenge studies to assess its immunoprotective capacity against each of the target bacterial pathogens. In parallel, future studies will be focused on optimizing the production of this antigen in edible crops that would allow for the straightforward formulation of oral vaccines, not requiring purification (Waheed et al. 2016); and assessing alternative immunization schemes to enhance the response against the FliC component.
Conclusion
LTBentero is a novel multiepitopic protein that can be functionally produced in the plant cell, having immunogenic activity in mice in terms of the induction of humoral systemic and mucosal antibody responses against a set of target epitopes upon oral and subcutaneous administration. Therefore, LTBentero is a promising oral immunogen with interesting implications in the fight against enteric diseases due to its low cost and easy administration.
References
Adkins I, Holubova J, Kosova M, Sadilkova L (2012) Bacteria and their toxins tamed for immunotherapy. Curr Pharm Biotechnol 13(8):1446–1473
Al-Barwani F, Donaldson B, Pelham SJ, Young SL, Ward VK (2014) Antigen delivery by virus-like particles for immunotherapeutic vaccination. Ther Deliv 5(11):1223–1240
Bass JJ, Wilkinson DJ, Rankin D, Phillips BE, Szewczyk NJ, Smith K, Atherton PJ (2016) An overview of technical considerations for Western blotting applications to physiological research. Scand J Med Sci Sports 27(1):4–25
Bergeron-Sandoval L, Girard A, Ouellet F, Archambault D, Sarhan F (2011) Production of human rotavirus and salmonella antigens in plants and eliCitation of fljb-specific humoral responses in mice. Mol Biotechnol 47:157–168
Bergman MA, Cummings LA, Alaniz RC, Mayeda L, Fellnerova I, Cookson BT (2005) CD4+-T-Cell responses generated during murine Salmonella enteric Serovar Typhimurium infection are directed towards multiple epitopes within the natural antigen FliC. Infect Immun 73:7226–7235
Broberg CA, Calder TJ, Orth K (2011) Vibrio parahaemolyticus cell biology and pathogenicity determinants. Microbes Infect 13(12–13):992–1001
Centers for Disease Control and Prevention (2008) Salmonella. http://www.cdc.gov/salmonella/. Accessed 27 May 2018
Chauhan N, Tiwari S, Iype T, Jain U (2017) An overview of adjuvants utilized in prophylactic vaccine formulation as immunomodulators. Expert Rev Vaccin 16(5):491–502
Chikwamba R, Cunnick J, Hathaway D, McMurray J, Mason H, Wang K (2002) A functional antigen in a practical crop: LT-B producing maize protects mice against Escherichia coli heat labile enterotoxin (LT) and cholera toxin (CT). Transgenic Res 11:479–493
Clements JD (1990) Construction of a nontoxic fusion peptide for immu-nization against Escherichia coli strains that produce heat-labil and heat-stable enterotoxins. Infect Immun 58:1159–1166
Cookson BT, Bevan MJ (1997) Identification of a natural T cell epitope presented by Salmonella-infected macrophages and recognized by T cells from orally immunized. Mice J Immunol 158:4310–4319
Cummings LA, Wilkerson WD, Bergsbaken T, Cookson BT (2006) In vivo fliC expression by Salmonella enterica serovar Typhimurium is heterogeneous, regulated by ClpX, and anatomically restricted. Mol Microbiol 61:795–809
Datta S, Janes E, Simonson JG (2008) Immunomagnetic separation and coagglutination of Vibrio parahaemolyticus with anti-flagellar protein monoclonal antibody. Clin Vaccine Immunol 15(10):1541–1546
Dellaporta SL, Wood J, Hicks JB (1983) A plant DNA minipreparation: version II. Plant Mol Biol Rep 1:19–21
Deng G, Zeng J, Jian M, Liu W, Zhang Z, Liu X, Wang Y (2013) Nanoparticulated heat-stable (STa) and heat-labile B subunit (LTB) recombinant toxin improves vaccine protection against enterotoxigenic Escherichia coli challenge in mouse. J Biosci Bioeng 115(2):147–153
Freinkman E, Okuda S, Ruiz N, Kahne D (2012) Regulated assembly of the transenvelope protein complex required for lipopolysaccharide export. Biochemistry 51:4800–4806
Girard MP, Steele D, Chaignat CL, Kieny MP (2006) A review of vaccine research and development: human enteric infections. Vaccine 24:2732–2750
Gordon MA, Graham SM, Walsh AL, Wilson L, Phiri A, Molyneux E (2008) Epidemics of invasive Salmonella enterica serovar enteritidis and S. enterica serovar Typhimurium infection associated with multidrug resistance among adults and children in Malawi. Clin Infect Dis 46:963–969
Hongli L, Xukui L, Ting L, Wensheng L, Lusheng S, Jin Z (2013) Transgenic tobacco expressed HPV16-L1 and LT-B combined immunization induces strong mucosal and systemic immune responses in mice. Hum Vaccin Immunother 9:83–89
Horsch RB, Fry JE, Hoffmann NL, Eichholtz D, Rogers SG, Fraley RT (1985) A simple and general method for transferring genes into plants. Science 227:1229–1231
Jacob CO, Sela M, Arnon R (1983) Antibodies against synthetic peptides of the B subunit of cholera toxin: crossreaction and neutralization of the toxin. Proc Natl Acad Sci 80(24):7611–7615
Jacob CO, Leitner M, Zamir A, Salomon D, Arnon R (1985) Priming immunization against cholera toxin and E. coli heat-labile toxin by a cholera toxin short peptide-beta-galactosidase hybrid synthesized in E. coli EMBO J 4(12):3339–3343
Kim SI, Veena JH, Gelvin SB (2007) Genome-wide analysis of AgrobacteriumT-DNA integration sites in the Arabidopsis genome generated under non-selective conditions. Plant J 51:779–791
Klipstein FA, Engert RF, Houghten RA (1986) Immunisation of volunteers with a synthetic peptide vaccine for enterotoxigenic Escherichia coli. Lancet 1:471–472
Koya V, Moayeri M, Leppla SH, Daniell H (2005) Plant-based vaccine: mice immunized with chloroplast-derived anthrax protective antigen survive anthrax lethal toxin challenge. Infect Immun 73(12):8266–8274
Lazorová L, Sjo¨lander A, Russell-Jones GJ, Linder J, Artursson P (1993) Intestinal tissue distribution and epithelial transport of the oral immunogen LTB, the B subunit of E. coli heat-labile enterotoxin. J Drug Target 1:331–340
Kremer CJ, O’Meara KM, Layton SL, Hargis BM, Cole K (2011) Evaluation of recombinant Salmonella expressing the flagellar protein fliC for persistence and enhanced antibody response in commercial turkeys. Poult Sci 90:752–758
Li C, Ye Z, Wen L, Chen R, Tian L, Zhao F, Pan j (2014) Identification of a novel vaccine candidate by immunogenic screening of Vibrio parahaemolyticus outer membrane proteins. Vaccine 32:6115–6121
Li X, Gu Y, Dong H, Wang W, Dong C (2015) Trapped lipopolysaccharide and LptD intermediates reveal lipopolysaccharide translocation steps across the Escherichia coli outer membrane. Sci Rep 5:11883
Liu M, Ruan X, Zhang C, Lawson SR, Knudsen DE, Nataro JP, Robertson DC, Zhang W (2011a) Heat-labile- and heat-stable-toxoid fusions (LTR192G-STaP13F) of human enterotoxigenic Escherichia coli elicit neutralizing antitoxin antibodies. Infect Immun 79:4002–4009
Liu R, Chen J, Li K, Zhang X (2011b) Identification and evaluation as a DNA vaccine candidate of a virulence-associated serine protease from a pathogenic Vibrio parahaemolyticus isolate. Fish Shellfish Immunol 30(6):1241–1248
Livingston B, Crimi C, Newman M, Higashimoto Y, Appella E, Sidney J, Sette A (2002) A rational strategy to design multiepitope immunogens based on multiple Th lymphocyte epitopes. J Immunol 168(11):5499–5506
Lun J, Xia C, Yuan C, Zhang Y, Zhong M, Huang T (2014) The outer membrane protein, LamB (maltoporin), is a versatile vaccine candidate among the Vibrio species. Vaccine 32(7):809–815
Majowicz SE, Musto J, Scallan E, Angulo FJ, Kirk M, O’Brien SJ (2010) The global burden of nontyphoidal Salmonella gastroenteritis. Clin Infect Dis 50:882–889
Martínez-Gonza´lez M, Rosales-Mendoza S, Soria-Guerra RE, Moreno-Fierros L, López-Revilla R, Korban SS, Guevara-Arauza JC, Alpuche-Solı´s AG (2011) Oral immunization with a lettuce-derived Escherichia coli heat-labile toxin B subunit induces neutralizing antibodies in mice. Plant Cell Tissue Org Cult 107:441–449
Mason HS, Haq TA, Clements JD, Arntzen CJ (1998) Edible vaccine protects mice against Escherichia coli heat-labile enterotoxin (LT): potatoes expressing a synthetic LT-B gene. Vaccine 16:1336–1343
McSorley SJ, Cookson BT, Jenkins MK (2000) Characterization of CD4+T cell responses during natural infection with Salmonella typhimurium. J Immunol 164:986–993
Nashar TO, Betteridge ZE, Mitchell RN (2001) Evidence for a role of ganglioside GM1 in antigen presentation: binding enhances presentation of Escherichia coli enterotoxin B subunit (EtxB) to CD4(+) T cells. Int Immunol 13(4):541–551
Newton SM, Jacob CO, Stocker BA (1989) Immune response to cholera toxin epitope inserted in Salmonella flagellin. Science 244(4900):70–72
Peng B, Ye JZ, Han Y, Zeng L, Zhang JY, Li H (2016) Identification of polyvalent protective immunogens from outer membrane proteins in Vibrio parahaemolyticus to protect fish against bacterial infection. Fish Shellfish Immunol 54:204–210
Pereira CM, Guth BE, Sbrogio-Almeida ME, Castilho BA (2001) Antibody response against Escherichia coli heat-stable enterotoxin expressed as fusions to flagellin. Microbiology 147:861–867
Rigano MM, Alvarez ML, Pinkhasov J, Jin Y, Sala F, Arntzen CJ, Walmsley AM (2004) Production of a fusion protein consisting of the enterotoxigenic Escherichia coli heat-labile toxin B subunit and a tuberculosis antigen in Arabidopsis thaliana. Plant Cell Rep 22:502–508
Ríos-Huerta R, Monreal-Escalante E, Govea-Alonso DO, Angulo C, Rosales-Mendoza S (2017) Expression of an immunogenic LTB-based chimeric protein targeting Zaire ebolavirus epitopes from GP1 in plant cells. Plant Cell Rep 36(2):355–365
Rosales-Mendoza S, Soria-Guerra RE, Lopez-Revilla R, Moreno-Fierros L, Alpuche-Solis AG (2008) Ingestion of transgenic carrots expressing the Escherichia coli heat-labile enterotoxin B subunit protects mice against cholera toxin challenge. Plant Cell Rep 27:79–84
Rosales-Mendoza S, Alpuche-Solís AG, Soria-Guerra RE, Moreno- Fierros L, Martínez-González L, Herrera-Díaz A, Korban SS (2009) Expression of an Escherichia coli antigenic fusion protein comprising the heat labile toxin B subunit and the heat stable toxin, and its assembly as a functional oligomer in transplastomic tobacco plants. Plant J 57:45–54
Rosales-Mendoza S, Soria-Guerra RE, Moreno-Fierros L, Govea-Alonso DO, Herrera-Díaz A, Korban SS, Alpuche-Solís AG (2011) Immunogenicity of nuclear-encoded LTB:ST fusion protein from Escherichia coli expressed in tobacco plants. Plant Cell Rep 30:1145–1152
Rosales-Mendoza S, Angulo C, Meza B (2016) Food-grade organisms as vaccine biofactories and oral delivery vehicles. Trends Biotechnol 34(2):124–136
Ruan X, Sack DA, Zhang W (2015) Genetic fusions of a CFA/I/II/IV MEFA (multiepitope fusión antigen) and a toxoid fusion of heat-stable toxin (STa) and heat-labile toxin (LT) of enterotoxigenic Escherichia coli (ETEC) retain broad anti-CFA and antitoxin antigenicity. PLoS ONE 10(3):e0121623
Ruiz N, Kahne D, Silhavy TJ (2009) Transport of lipopolysaccharide across the cell envelope: the long road of discovery. Nat Rev Microbiol 7:677–683
Saarilahti HT, Palva ET, Holmgren J, Sanchez J (1989) Fusion of genes encoding Escherichia coli heat-stable enterotoxin and outer membrane protein OmpC. Infect Immun 57:3663–3665
Scotti N, Rybicki EP (2013) Virus-like particles produced in plants as potential vaccines. Expert Rev Vaccin 12(2):211–224
Tacket CO, Mason HS, Losonsky G, Clements JD, Levine MM, Arntzen CJ (1998) Immunogenicity in humans of a recombinant bacterial antigen delivered in a transgenic potato. Nat Med 4:607–609
Takeyama N, Kiyono H, Yuki Y (2015) Plant-based vaccines for animals and humans: recent advances in technology and clinical trials. Ther Adv Vaccin 3:139–154
Waheed MT, Sameeullah M, Khan FA, Syed T, Ilahi M, Gottschamel J, Lössl AG (2016) Need of cost-effective vaccines in developing countries: what plant biotechnology can offer? Springerplus 5:65
Walker R, Steele D, Aguado T (2007) Review: Analysis of strategies to successfully vaccinate infants in developing countries against enterotoxigenic E. coli (ETEC) disease. Vaccine 25:2545–2566
Wang N, Yang Z, Zang M, Liu Y, Lu C (2013) Identification of Omp38 by immunoproteomic analysis and evaluation as a potential vaccine antigen against Aeromonas hydrophila in Chinese breams. Fish Shellfish Immunol 34(1):74–81
Walmsley AM, Alvarez ML, Jin Y, Kirk DD, Lee SM, Pinkhasov J, Rigano MM, Arntzen CJ, Mason HS (2003) Expression of the B subunit of Escherichia coli heat-labile enterotoxin as a fusion protein in transgenic tomato. Plant Cell Rep 21:1020–1026
WHO (2010) Cholera vaccines: WHO position paper. Wkly Epidemiol Rec 85:117–128
WHO (2017) Diarrhoeal disease: https://www.who.int/news-room/factsheets/detail/diarrhoeal-disease. Accessed 16 July 2018
Xiang QJ, Wang HY, Wang ZS, Zhang YZ, Dong CJ (2014) Characterization of lipopolysaccharide transport protein complex. Cent Eur J Biol 9:131–138
Yamamoto M, McGhee JR, Hagiwara Y, Otake S, Kiyono H (2001) Genetically manipulated bacterial toxin as a new generation mucosal adjuvant. Scand J Immunol 53(3):211–217
You J, Xu Y, He M, McAllister TA, Thacker PA, Li X, Wang T, Jin L (2011) Protection of mice against enterotoxigenic E. coli by immunization with a polyvalent enterotoxin comprising a combination of LTB, STa, and STb. Appl Microbiol Biotechnol 89:1885–1893
Zha Z, Li C, Li W, Ye Z, Pan J (2016) LptD is a promising vaccine antigen and potential immunotherapeutic target for protection against Vibrio species infection. Nat Sci Rep 6:38577
Zhang W, Zhang C, Francis DH, Fang Y, Knudsen D, Nataro JP, Robertson DC (2010) Genetic fusions of heat-labile (LT) and heat-stable (ST) toxoids of porcine enterotoxigenic Escherichia coli elicit neutralizing anti-LT and anti-STa antibodies. Infect Immun 78:316–325
Zheng JP, Zhang ZS, Li SQ, Liu XX, Yuan SL, Wang P, Zhan DW, Wang LC, Huang CF (2005) Construction of a novel Shigella live-vector strain coexpressing CS3 and LTB/STm of enterotoxigenic E. coli. World J Gastroenterol 11:3411–3418
Acknowledgements
We thank Dr. Dania Govea for technical support. Current investigations from the group are supported by Consejo Nacional de Ciencia y Tecnología (CONACYT) Grants INFR-2016-271182 and CB-256063 to Sergio Rosales Mendoza and CB-2010-01-151818 and INFR- 2014-01-225924 to Carlos Angulo.
Author information
Authors and Affiliations
Contributions
CA and SR-M conceived the research and designed the experiments. ET conducted the experiments and analyzed the data. ET, SR-M and CA wrote the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Trujillo, E., Rosales-Mendoza, S. & Angulo, C. A multi-epitope plant-made chimeric protein (LTBentero) targeting common enteric pathogens is immunogenic in mice. Plant Mol Biol 102, 159–169 (2020). https://doi.org/10.1007/s11103-019-00938-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11103-019-00938-3