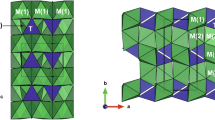
Abstract
The influence of pentavalent arsenic and phosphorus on crystal chemical features of the feldspar framework was studied on the minerals belonging to the sanidine–filatovite solid-solution series from fumarolic exhalations of the Tolbachik volcano (Kamchatka, Russia). The reported feldspars demonstrate the first example of natural continuous solid solution between silicate and arsenate. The content of As2O5 varies from 0.0 to 45 wt% along with the highest phosphorus content found in natural feldspars: up to 5 wt% P2O5. The major substitution schemes in the studied feldspars are Al3+ + M5+ → 2Si4+ and M2+ + M5+ → Al3+ + Si4+, where M2+ = Zn and Cu and M5+ = As and P. The crystal structures of four samples with empirical formulae K0.95[(Si2.98Al0.93Fe0.08As0.02)Ʃ4.01O8] (space group C2/m), (K0.91Na0.06Ca0.03)Ʃ1.00[(Si2.81Al1.14As0.03P0.03Fe0.01)Ʃ4.02O8] (C2/m), K0.82[(Al1.92As1.11Si0.89Zn0.05Fe0.03Cu0.02P0.01)Ʃ4.03O8] (I2/c), K0.99[(Si1.87Al1.51As0.53P0.05Fe0.02Cu0.01)Ʃ3.99O8] (I2/c) were studied. The phase transition in the sanidine–filatovite solid-solution series is C2/m → I2/c. This is caused by the change of the Al:(Si + As) ratio from 1:3 in sanidine to 1:1 in filatovite along with significant ordering of Al, Si and As in the framework.








Similar content being viewed by others
References
Aliatis et al (2015) A comparison between ab initio calculated and measured Raman spectrum of triclinic albite (NaAlSi3O8). J Raman Spectr 46:501–508
Bendel V, Schmidt BC (2008) Raman spectroscopic characterisation of disordered alkali feldspars along the join KAlSi3O8–NaAlSi3O8: application to natural sanidine and anorthoclase. Eur J Mineral 20:1055–1065
Bokiy GB, Borutsky BE (eds) (2003) Minerals. Reference book. Vol. V: Tectosilicates. Pt. 1: Silicates with Interrupted Frameworks and Feldspars. Nauka Publishing, Moscow. (in Russian)
Brown WL, Parsons I (1989) Alkali feldspars: ordering rates, phase transformations and behavior diagrams for igneous rocks. Miner Mag 53:25–42
Britvin SN, Dolivo-Dobrovolsky DV, Krzhizhanovskaya MG (2017) Software for processing the X-ray powder diffraction data obtained from the curved image plate detector of Rigaku RAXIS Rapid II diffractometer. Zapiski RMO 146(3):104–107 (in Russian)
Broska I, Williams CT, Uher P, Konecny P, Leichmann J (2004) The geochemistry of phosphorus in different granite suites of the Western Carpathians, Slovakia: the role of apatite and P-bearing feldspar. Chem Geol 205:1–15
Christy AG (2018) Quantifying lithophilicity, chalcophilicity and siderophilicity. Eur J Mineral 30:193–204
Deer WA, Howie RA, Zussman J (2001) Rock-Forming minerals. In: Framework silicates: feldspars. The Geological Society, London
Ferguson RB, Ball NA, Cerny P (1991) Structure refinement of an adularian end-member high sanidine from the Buck Claim pegmatite, Bernic Lake, Manitoba. Can Miner 29:543–552
Filatov SK, Krivovichev SV, Burns PC, Vergasova LP (2004) Crystal structure of filatovite, K(Al, Zn)2(As, Si)2O8, the first arsenate of the feldspar group. Eur J Mineral 16:537–543
Finney JJ, Bailey SW (1964) Crystal structure of an authigenic maximum microcline. Z Kristallogr 119:413–426
Freeman JJ, Wang A, Kuebler KE, Jolliff BL, Haskin LA (2008) Characterization of natural feldspars by Raman spectroscopy for future planetary exploration. Can Mineral 46:1477–1500
Fryda J, Breiter K (1995) Alkali feldspars as a main phosphorus reservoirs in rare-metal granites: three examples from the Bohemian massif (Czech Republic). Terra Nova 7:315–320
Gagné OC, Hawthorne FC (2015) Comprehensive derivation of bond-valence parameters for ion pairs involving oxygen. Acta Cryst B71:562–578
Grew ES, Yates MG, Belakovskiy DI, Rouse RC, Su SC, Marquez N (1994) Hyalotekite from reedmergnerite-bearing peralkaline pegmatite, Dara-i-Pioz, Tajikistan and from Mn skarn, Långban, Värmland, Sweden: a new look at an old mineral. Mineral Mag 58:285–297
Griffen DA, Ribbe PH (1976) Refinement of the crystal structure of celsian. Am Mineral 61:414–418
Gualtieri AF (2000) Accuracy of XRPD QPA using the combined Rietveld-RIR method. J Appl Cryst 33:267–278
Kontak DJ, Martin RF, Richard L (1996) Patterns in phosphorus enrichment in alkali feldspar, South Mountain batholith, Nova Scotia, Canada. Eur J Mineral 8:805–824
Kotelnikov AR, Ananiev VV, Kovalsky AM, Suk NI (2011) Synthesis of phosphorus- and arsenic-bearing framework silicates similar to feldspar. Vest Nauk Zem RAN 3:6047
Kroll H, Bambauer HV, Schirmer V (1980) The high albite-monalbite and analbite-monalbite transitions. Am Mineral 65:1192–1211
Kroll H, Ribbe PH (1987) Determining (Al, Si) distribution and strain in alkali feldspars using lattice parameters and diffraction-peak positions: a review. Am Mineral 72(5–6):491–506
Kuehner SM, Joswiak DJ (1996) Naturally occurring ferric iron sanidine from the Leucite Hills lamproite. Am Mineral 81:229–237
Lacroix A (1912) Note pre´liminaire sur quelques mine´raux de Madagascar dont plusieurs peuvent tre utilise´s comme gemmes. Com Renl’Ac Sci Paris 155:672–677
Laves F (1960) Al/Si-Verteilungen, Phasen-Transformationen und Namen der Alkalifeldspate. Z Kristallogr 113:265–296
Linthout K, Lustenhouwer W (1993) Ferrian high sanidine in a lamproite from Cancarix, Spain. Mineral Mag 57:289–299
London D, Cerny P, Loomis JL, Pan JJ (1990) Phosphorus in alkali feldspars of rare-element granitic pegmatites. Can Miner 28:771–786
London D (1992) Phosphorus in S-type magmas: the P2O5 content of feldspars from peraluminous granites, pegmatites and rhyolites. Am Mineral 77:126–145
London D, Wolf MB, Morgan GB, Garrido MG (1999) Experimental silicate-phosphate equilibria in peraluminous granitic magmas, with a case study of the Alburquerque batholith at Tres Arroyos Badajoz, Spain. J Petrol 40:215–240
Löwenstein W (1954) The distribution of aluminium in the tetrahedra of silicates and aluminates. Am Mineral 39:92–96
Malcherek T, Carpenter MA, Kroll H, Salje EKH (1999) Cation ordering in BaAl2Ge2O8-feldspar: implications for the I-1 C-1 phase transition in anorthite. Phys Chem Miner 26:354–366
McKeown DA (2005) Raman spectroscopy and vibrational analyses of albite: from 25°C through the melting temperature. Am Mineral 90:1506–1517
McConnel JDC (1971) Electron optical study of phase transformations. Mineral Mag 38:1–20
Megaw HD (1973) Crystal Structures: a Working Approach. W.B. Saunders Co., London
Megaw HD (1974) The architecture of the feldspars. In: MacKenzie WS, Zussman J (eds) The Feldspars. Manchester University Press, Manchester
Onorato E, Penta M, Sgarlata F (1963) Struttura del sanidino. Per Miner 32:1–34
Parsons I (ed) (1994) Feldspars and their reactions. Proceedings of the NATO Advanced Study Institute on Feldspars and Their Reactions Edinburgh, United Kingdom
Pekov IV, Koshlyakova NN, Zubkova NV, Lykova IS, Britvin SN, Yapaskurt VO, Agakhanov AA, Shchipalkina NV, Turchkova AG, Sidorov EG (2018) Fumarolic arsenates—a special type of arsenic mineralization. Eur J Mineral 30(2):305–322
Petriček V, Duŝek M, Palatinus L (2014) Crystallographic computing system JANA2006: general features. Z Kristallogr 229(5):345–352
Ribbe PH (1983) The chemistry, structure and nomenclature of feldspars. Rev Mineral 2:1–20
Salje E (1985) Thermodynamics of sodium feldspar: 1. Order parameter treatment and strain induced coupling effects. Phys Chem Miner 12:93–98
Scambos TA, Smyth JR, McCormick TC (1987) Crystal-structure refinement of high sanidine from the upper mantle. Am Mineral 72:973–978
Serafimova EK (1992) Mineral paragenesis of volcanic exhalations. Post-Eruptive Mineral Formation on Active Volcanoes of Kamchatka. P I. Far Eastern Branch of RAS, Vladivostok, 31–52 (in Russian)
Shannon RD, Prewitt CT (1969) Effective ionic radii in oxides and fluorides. Acta Crystal B25:925–946
Shchipalkina NV, Pekov IV, Zubkova NV, Koshlyakova NN, Sidorov EG (2019) Natural forsterite strongly enriched by arsenic and phosphorus: chemistry, crystal structure, crystal morphology and zonation. Phys Chem Mineral 46:889–898
Simpson DR (1977) Aluminium phosphate variants of feldspar. Am Mineral 62:351–355
Smith JV, Brown WL (1988) Feldspar minerals 1 crystal structures, physical, chemical and microstructural properties. Springer, Berlin
Tribaudino M, Benna P, Bruno E (1998) Structural variations induced by thermal treatment in lead feldspar (PbAl2Si2O8). Am Mineral 83:159–166
Vergasova LP, Filatov SK (2016) A study of volcanogenic exhalation mineralization. J Volcanol Seismol 10:71–85
Vergasova LP, Krivovichev SV, Britvin SN, Burns PC, Ananiev VV (2004) Filatovite, K[(Al, Zn)2(As, Si)2O8], a new mineral species from the Tolbachik volcano, Kamchatka peninsula, Russia. Eur J Mineral 16:533–536
Weitz G (1972) Die Struktur des Sanidins bei verschiedenen Ordnungsgraden. Z Kristallogr 136:418–426
Yazdani MR, Tuurijarvi T, Bhatnagar A, Vahala R (2016) Adsorptive removal of arsenic (V) from aqueous phase by feldspars: kinetics, mechanism, and thermodynamic aspects of adsorption. J Mol Liq 214:149–156
Yazdani MR, Bhatnagar A, Vahala R (2017) Synthesis, characterization and the exploration of nano-TiO2/feldspar-embedded chitosan beads towards UV-assisted adsorptive abatement of aqueous arsenic (As). Chem Eng J 316:370–382
Acknowledgements
We thank two anonymous referees for valuable comments. This work was supported by the Russian Science Foundation, Grant no. 19–17-00050. Powder XRD study was carried out with the technical support by the SPbSU X-Ray Diffraction Resource Center.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shchipalkina, N.V., Pekov, I.V., Britvin, S.N. et al. Arsenic and phosphorus in feldspar framework: sanidine–filatovite solid solution series from fumarolic exhalations of the Tolbachik volcano, Kamchatka, Russia. Phys Chem Minerals 47, 1 (2020). https://doi.org/10.1007/s00269-019-01067-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00269-019-01067-5