Abstract
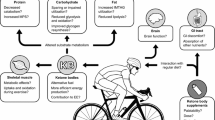
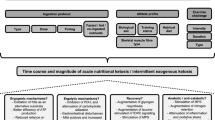
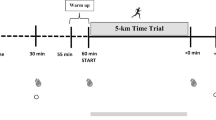
Ketone bodies (KB) provide an alternative energy source and uniquely modulate substrate metabolism during endurance exercise. Nutritional ketosis (blood KBs > 0.5 mM) can be achieved within minutes via exogenous ketone supplementation or days-to-weeks via conforming to a very low-carbohydrate, ketogenic diet (KD). In contrast to short-term (< 2 weeks) KD ingestion, chronic adherence (> 3 weeks) leads to a state of keto-adaptation. However, despite elevating blood KBs to similar concentrations, exogenous ketone supplementation and keto-adaptation are not similar metabolic states as they elicit diverse and distinct effects on substrate availability and metabolism during exercise; meaning that their influence on endurance exercise performance is different. In contrast to contemporary, high(er)-carbohydrate fuelling strategies, inducing nutritional ketosis is rarely ergogenic irrespective of origin and, in fact, can impair endurance performance. Nonetheless, exogenous ketone supplementation and keto-adaptation possess utility for select endurance events and individuals, thus warranting further research into their performance effects and potential strategies for their optimisation. It is critical, however, that future research considers the limitations of measuring blood KB concentrations and their utilisation, and assess the effect of nutritional ketosis on performance using exercise protocols reflective of real-world competition. Furthermore, to reliably assess the effects of keto-adaptation, rigorous dietary-training controls of sufficient duration should be prioritised.


Similar content being viewed by others
References
Romijn JA, Coyle EF, Sidossis LS, et al. Regulation of endogenous fat and carbohydrate metabolism in relation to exercise intensity and duration. Am J Physiol. 1993;265:E380–91.
Robinson AM, Williamson DH. Physiological roles of ketone bodies as substrates and signals in mammalian tissues. Physiol Rev. 1980;60:143–87.
Evans M, Cogan KE, Egan B. Metabolism of ketone bodies during exercise and training: physiological basis for exogenous supplementation. J Physiol. 2016;595:2857–71. https://doi.org/10.1113/JP273185.
Volek JS, Noakes T, Phinney SD. Rethinking fat as a fuel for endurance exercise. Eur J Sport Sci. 2015;15:13–20. https://doi.org/10.1080/17461391.2014.959564.
Pardridge WM (1991) Blood–brain barrier transport of glucose, free fatty acids, and ketone bodies. In: Fuel homeostasis and the nervous system. Advances in experimental medicine and biology. Oxygen Transport to Tissue Xxxiv, pp 43–53.
Halestrap AP, Wilson MC. The monocarboxylate transporter family—role and regulation. IUBMB Life. 2012;64:109–19. https://doi.org/10.1002/iub.572.
Shaw DM, Merien F, Braakhuis A, et al. Effect of a ketogenic diet on submaximal exercise capacity and efficiency in runners. Med Sci Sport Exerc. 2019. https://doi.org/10.1249/MSS.0000000000002008.
Phinney SD, Bistrian BR, Evans WJ, et al. The human metabolic response to chronic ketosis without caloric restriction: preservation of submaximal exercise capability with reduced carbohydrate oxidation. Metabolism. 1983;32:769–76.
Burke LM, Ross ML, Garvican-Lewis LA, et al. Low Carbohydrate, High Fat diet impairs exercise economy and negates the performance benefit from intensified training in elite race walkers. J Physiol. 2016;595:1–23. https://doi.org/10.1113/JP273230.
Volek JS, Freidenreich DJ, Saenz C, Kunces LJ. Metabolic characteristics of keto-adapted ultra-endurance runners. Metabolism. 2015;65:100–10.
Webster CC, Noakes TD, Chacko SK, et al. Gluconeogenesis during endurance exercise in cyclists habituated to a long-term low carbohydrate high fat diet. J Physiol. 2016;594:4389–405. https://doi.org/10.1113/JP271934.
Cahill GF. Fuel metabolism in starvation. Annu Rev Nutr. 2006;26:1–22. https://doi.org/10.1146/annurev.nutr.26.061505.111258.
Owen OE, Morgan AP, Kemp HG, et al. Brain metabolism during fasting. J Clin Invest. 1967;46:1589–95. https://doi.org/10.1172/JCI105650.
McSwiney FT, Wardrop B, Hyde PN, et al. Keto-adaptation enhances exercise performance and body composition responses to training in endurance athletes. Metab Clin Exp. 2018;81:25–34. https://doi.org/10.1016/j.metabol.2017.10.010.
Dostal T, Plews DJ, Hofmann P, et al. Effects of a 12-week very-low carbohydrate high-fat diet on maximal aerobic capacity, high-intensity intermittent exercise, and cardiac autonomic regulation: non-randomized parallel-group study. Front Physiol. 2019;10:1–12. https://doi.org/10.3389/fphys.2019.00912.
Sherrier M, Li H. The impact of keto-adaptation on exercise performance and the role of metabolic-regulating cytokines. Am J Clin Nutr. 2019;67:789-12. https://doi.org/10.1093/ajcn/nqz145.
Phinney SD (2004) Ketogenic diets and physical performance. Nutr Metab.
Murtaza N, Burke L, Vlahovich N, et al. The effects of dietary pattern during intensified training on stool microbiota of elite race walkers. Nutrients. 2019;11:261-14. https://doi.org/10.3390/nu11020261.
Murtaza N, Burke LM, Vlahovich N, et al. Analysis of the effects of dietary pattern on the oral microbiome of elite endurance athletes. Nutrients. 2019;11:614. https://doi.org/10.3390/nu11030614.
McKay AKA, Peeling P, Pyne DB, et al. Chronic adherence to a ketogenic diet modifies iron metabolism in elite athletes. Med Sci Sport Exerc. 2019;51:548–55. https://doi.org/10.1249/MSS.0000000000001816.
McKay AKA, Peeling P, Pyne DB, et al. Acute carbohydrate ingestion does not influence the post-exercise iron-regulatory response in elite keto-adapted race walkers. J Sci Med Sport. 2019. https://doi.org/10.1016/j.jsams.2018.12.015.
Carr A, Sharma AP, Ross ML, et al. Chronic ketogenic low carbohydrate high fat diet has minimal effects on acid-base status in elite athletes. Nutrients. 2018;10:1–13. https://doi.org/10.3390/nu10020236.
McKay AKA, Pyne DB, Peeling P, et al. The impact of chronic carbohydrate manipulation on mucosal immunity in elite endurance athletes. J Sports Sci. 2018;37:553–9. https://doi.org/10.1080/02640414.2018.1521712.
Coggan AR, Coyle EF. Reversal of fatigue during prolonged exercise by carbohydrate infusion or ingestion. J Appl Physiol. 1987;63:2388–95.
Gonzalez JT, Fuchs CJ, Smith FE, et al. Ingestion of glucose or sucrose prevents liver but not muscle glycogen depletion during prolonged endurance-type exercise in trained cyclists. Am J Physiol Endocrinol Metab. 2015;309:E1032–9. https://doi.org/10.1152/ajpendo.00376.2015.
Coyle EF, Coggan AR, Hemmert MK, Ivy JL. Muscle glycogen utilization during prolonged strenuous exercise when fed carbohydrate. J Appl Physiol. 1986;61:165–72.
Burke LM. “Fat adaptation” for athletic performance: the nail in the coffin? J Appl Physiol. 2006;100:7–8. https://doi.org/10.1152/japplphysiol.01238.2005.
Yeo WK, Carey AL, Burke L, et al. Fat adaptation in well-trained athletes: effects on cell metabolism. Appl Physiol Nutr Metab. 2011;36:12–22. https://doi.org/10.1139/H10-089.
Spriet LL. New insights into the interaction of carbohydrate and fat metabolism during exercise. Sports Med. 2014;44:87–96. https://doi.org/10.1007/s40279-014-0154-1.
Burke LM. Re-examining high-fat diets for sports performance: did we call the “Nail in the Coffin” too soon? Sports Med. 2015;45(Suppl 1):S33–49. https://doi.org/10.1007/s40279-015-0393-9.
Mirtschin JG, Forbes SF, Cato LE, et al. Organisation of dietary control for nutrition-training intervention involving periodized carbohydrate (CHO) availability and ketogenic low CHO high fat (LCHF) diet. Int J Sport Nutr Exerc Metab. 2018;28:480–9. https://doi.org/10.1123/ijsnem.2017-0249.
Jeukendrup AE, Wallis GA. Measurement of substrate oxidation during exercise by means of gas exchange measurements. Int J Sports Med. 2005;26:S28–37. https://doi.org/10.1055/s-2004-830512.
Rowlands DS. Model for the behaviour of compartmental CO2 stores during incremental exercise. Eur J Appl Physiol. 2005;93:555–68. https://doi.org/10.1007/s00421-004-1217-z.
Pinckaers PJM, Churchward-Venne TA, Bailey D, van Loon LJC. Ketone bodies and exercise performance: the next magic bullet or merely hype? Sports Med. 2016;47:383–91. https://doi.org/10.1007/s40279-016-0577-y.
Cox PJ, Clarke K. Acute nutritional ketosis: implications for exercise performance and metabolism. Extrem Physiol Med. 2014;3:17.
Margolis LM, O’Fallon KS. Utility of ketone supplementation to enhance physical performance: a systematic review. Adv Nutr. 2019;31:834–8. https://doi.org/10.1093/advances/nmz104.
Evans M, Patchett E, Nally R, et al. Effect of acute ingestion of β-hydroxybutyrate salts on the response to graded exercise in trained cyclists. Eur J Sport Sci. 2018;45:1–11. https://doi.org/10.1080/17461391.2017.1421711.
O’Malley T, Myette-Cote E, Durrer C, Little JP. Nutritional ketone salts increase fat oxidation but impair high-intensity exercise performance in healthy adult males. Appl Physiol Nutr Metab. 2017;42:1031–5. https://doi.org/10.1139/apnm-2016-0641.
Rodger S, Plews D, Laursen P, Driller M. Oral β-hydroxybutyrate salt fails to improve 4-minute cycling performance following submaximal exercise. J Sci Cycling. 2017;6:26–31.
Stubbs BJ, Cox PJ, Evans R, et al. On the metabolism of exogenous ketones in humans. Front Physiol. 2017;8:848. https://doi.org/10.3389/fphys.2017.00848.
Waldman HS, Basham SA, Price FG, et al. Exogenous ketone salts do not improve cognitive responses after a high-intensity exercise protocol in healthy college-aged males. Appl Physiol Nutr Metab. 2018;43:711–7. https://doi.org/10.1139/apnm-2017-0724.
Veech RL. The therapeutic implications of ketone bodies: the effects of ketone bodies in pathological conditions: ketosis, ketogenic diet, redox states, insulin resistance, and mitochondrial metabolism. Prostaglandins Leukot Essent Fatty Acids. 2004;70:309–19. https://doi.org/10.1016/j.plefa.2003.09.007.
Stubbs BJ, Cox PJ, Kirk T, et al. Gastrointestinal effects of exogenous ketone drinks are infrequent, mild and vary according to ketone compound and dose. Int J Sport Nutr Exerc Metab. 2019. https://doi.org/10.1123/ijsnem.2019-0014.
Scott BE, Laursen PB, James LJ, et al. The effect of 1,3-butanediol and carbohydrate supplementation on running performance. J Sci Med Sport. 2018. https://doi.org/10.1016/j.jsams.2018.11.027.
Shaw DM, Merien F, Braakhuis A, et al. The effect of 1,3-butanediol on cycling time-trial performance. Int J Sport Nutr Exerc Metab. 2019. https://doi.org/10.1123/ijsnem.2018-0284.
Puchowicz MA, Smith CL, Bomont C, et al. Dog model of therapeutic, ketosis induced by oral administration of R, S-1,3-butanediol diacetoacetate. J Nutr Biochem. 2000;11:281–7. https://doi.org/10.1016/S0955-2863(00)00079-6.
Desrochers S, David F, Garneau M, et al. Metabolism of R- and S-1,3-butanediol in perfused livers from meal-fed and starved rats. Biochem J. 1992;285:647–53.
Desrochers S, Quinze K, Dugas H, et al. R, S-1,3-butanediol acetoacetate esters, potential alternates to lipid emulsions for total parenteral nutrition. J Nutr Biochem. 1995;6:111–8. https://doi.org/10.1016/0955-2863(94)00011-A.
Mehlman MA, Tobin RB, Hahn HK, et al. Metabolic fate of 1,3-butanediol in the rat: liver tissue slices metabolism. J Nutr. 1971;101:1711–8.
Tate RL, Mehlman MA, Tobin RB. Metabolic fate of 1,3-butanediol in rat—conversion to beta-hydroxybutyrate. J Nutr. 1971;101:1719–26.
Desrochers S, Dubreuil P, Brunet J, et al. Metabolism of (R, S)-1,3-butanediol acetoacetate esters, potential parenteral and enteral nutrients in conscious pigs. Am J Physiol. 1995;31:E660–7.
D’agostino DP, Pilla R, Held HE, et al. Therapeutic ketosis with ketone ester delays central nervous system oxygen toxicity seizures in rats. Am J Physiol Regul Integr Comp Physiol. 2013;304:R829–36. https://doi.org/10.1152/ajpregu.00506.2012.
Leckey JJ, Ross ML, Quod M, et al. Ketone diester ingestion impairs time-trial performance in professional cyclists. Front Physiol. 2017;8:806. https://doi.org/10.3389/fphys.2017.00806.
Clarke K, Tchabanenko K, Pawlosky R, et al. Kinetics, safety and tolerability of (R)-3-hydroxybutyl (R)-3-hydroxybutyrate in healthy adult subjects. Regul Toxicol Pharmacol. 2012;63:401–8. https://doi.org/10.1016/j.yrtph.2012.04.008.
Cox PJ, Kirk T, Ashmore T, et al. Nutritional ketosis alters fuel preference and thereby endurance performance in athletes. Cell Metab. 2016;24:256–68. https://doi.org/10.1016/j.cmet.2016.07.010.
Clarke K, Tchabanenko K, Pawlosky R, et al. Oral 28-day and developmental toxicity studies of (R)-3-hydroxybutyl (R)-3-hydroxybutyrate. Regul Toxicol Pharmacol. 2012;63:196–208. https://doi.org/10.1016/j.yrtph.2012.04.001.
Shivva V, Cox PJ, Clarke K, et al. The population pharmacokinetics of d-β-hydroxybutyrate following administration of (R)-3-Hydroxybutyl (R)-3-hydroxybutyrate. AAPS J. 2016;18:678–88. https://doi.org/10.1208/s12248-016-9879-0.
Myette-Cote E, Neudorf H, Rafiei H, et al. Prior ingestion of exogenous ketone monoester attenuates the glycemic response to an oral glucose tolerance test in healthy young individuals. J Physiol. 2018;596:1385–95. https://doi.org/10.1113/JP275709.
Stubbs BJ, Cox PJ, Evans RD, et al. A ketone ester drink lowers human ghrelin and appetite. Obesity. 2017;26:269–73. https://doi.org/10.1002/oby.22051.
Neudorf H, Durrer C, Myette-Cote E, et al. Oral ketone supplementation acutely increases markers of NLRP3 inflammasome activation in human monocytes. Mol Nutr Food Res. 2019. https://doi.org/10.1002/mnfr.201801171.
Evans M, Egan B. Intermittent running and cognitive performance after ketone ester ingestion. Med Sci Sport Exerc. 2018;50:2330–8. https://doi.org/10.1249/MSS.0000000000001700.
Faull OK. Beyond RPE: the perception of exercise under normal and ketotic conditions. Front Physiol. 2019;10:229. https://doi.org/10.3389/fphys.2019.00229.
Dearlove DJ, Faull OK, Rolls E, et al. Nutritional ketoacidosis during incremental exercise in healthy athletes. Front Physiol. 2019;10:290. https://doi.org/10.3389/fphys.2019.00290.
Vandoorne T, De Smet S, Ramaekers M, et al. Intake of a ketone ester drink during recovery from exercise promotes mTORC1 signalling but not glycogen resynthesis in human muscle. Front Physiol. 2017;8:310. https://doi.org/10.3389/fphys.2017.00310/full.
Holdsworth DA, Cox PJ, Kirk T, et al. A ketone ester drink increases postexercise muscle glycogen synthesis in humans. Med Sci Sport Exerc. 2017;49:1789–95. https://doi.org/10.1249/MSS.0000000000001292.
Poffé C, Ramaekers M, Van Thienen R, Hespel P. Ketone ester supplementation blunts overreaching symptoms during endurance training overload. J Physiol. 2019. https://doi.org/10.1113/JP277831.
Van Gelder J, Shafiee M, De Clercq E, et al. Species-dependent and site-specific intestinal metabolism of ester prodrugs. Int J Pharm. 2000;205:93–100. https://doi.org/10.1016/S0378-5173(00)00507-X.
Tsai Y-C, Liao T-H, Lee J-A. Identification of L-3-hydroxybutyrate as an original ketone body in rat serum by column-switching high-performance liquid chromatography and fluorescence derivatization. Analytical Biochemistry. 2003;319:34–41. https://doi.org/10.1016/S0003-2697(03)00283-5.
Tsai Y-C, Chou Y-C, Wu A-B, et al. Stereoselective effects of 3-hydroxybutyrate on glucose utilization of rat cardiomyocytes. Life Sci. 2006;78:1385–91. https://doi.org/10.1016/j.lfs.2005.07.013.
Lincoln BC, Rosiers CD, Brunengraber H. Metabolism of S-3-hydroxybutyrate in the perfused rat liver. Arch Biochem Biophys. 1987;259:149–56. https://doi.org/10.1016/0003-9861(87)90480-2.
Webber RJ. Utilization of L(+)-3-hydroxybutyrate, D(−)-3-hydroxybutyrate, acetoacetate, and glucose for respiration and lipid synthesis in the 18-day-old rat. J Biol Chem. 1977;252:5222–6.
Misra S, Oliver NS. Utility of ketone measurement in the prevention, diagnosis and management of diabetic ketoacidosis. Diabet Med. 2015;32:14–23. https://doi.org/10.1111/dme.12604.
Urbain P, Bertz H. Monitoring for compliance with a ketogenic diet: what is the best time of day to test for urinary ketosis? Nutr Metab. 2016;13:77. https://doi.org/10.1186/s12986-016-0136-4.
Ceriotti F, Kaczmarek E, Guerra E, et al. Comparative performance assessment of point-of-care testing devices for measuring glucose and ketones at the patient bedside. J Diabetes Sci Technol. 2015;9:268–77. https://doi.org/10.1177/1932296814563351.
Guimont M-C, Desjobert H, Fonfrède M, et al. Multicentric evaluation of eight glucose and four ketone blood meters. Clin Biochem. 2015;48:1310–6. https://doi.org/10.1016/j.clinbiochem.2015.07.032.
Thomas C, Bishop DJ, Lambert K, et al. Effects of acute and chronic exercise on sarcolemmal MCT1 and MCT4 contents in human skeletal muscles: current status. Am J Physiol Regul Integr Comp Physiol. 2012;302:R1–14. https://doi.org/10.1152/ajpregu.00250.2011.
Winder WW, Holloszy JO, Baldwin KM. Enzymes involved in ketone utilization in different types of muscle: adaptation to exercise. Eur J Biochem. 1974;47:461–7. https://doi.org/10.1111/j.1432-1033.1974.tb03713.x.
Romijn JA, Coyle EF, Hibbert J, Wolfe RR. Comparison of indirect calorimetry and a new breath 13C/12C ratio method during strenuous exercise. Am J Physiol. 1992;263:E64–71.
Frayn KN. Calculation of substrate oxidation rates in vivo from gaseous exchange. J Appl Physiol Respir Environ Exerc Physiol. 1983;55:628–34.
Soeters MR, Serlie MJ, Sauerwein HP, et al. Characterization of D-3-hydroxybutyrylcarnitine (ketocarnitine): an identified ketosis-induced metabolite. Metab Clin Exp. 2012;61:966–73. https://doi.org/10.1016/j.metabol.2011.11.009.
Féry F, Balasse EO. Effect of exercise on the disposal of infused ketone bodies in humans. J Clin Endocrinol Metab. 1988;67:245–50. https://doi.org/10.1210/jcem-67-2-245.
Hawley JA, Leckey JJ. Carbohydrate dependence during prolonged, intense endurance exercise. Sports Med. 2015;45(Suppl 1):S5–12. https://doi.org/10.1007/s40279-015-0400-1.
Gejl KD, Thams L, Hansen M, et al. No superior adaptations to carbohydrate periodization in elite endurance athletes. Med Sci Sport Exerc. 2017;49:2486–97. https://doi.org/10.1249/MSS.0000000000001377.
Krogh A, Lindhard J. The relative value of fat and carbohydrate as sources of muscular energy: with appendices on the correlation between standard metabolism and the respiratory quotient during rest and work. Biochem J. 1920;14:290–363.
Ørtenblad N, Nielsen J. Muscle glycogen and cell function—location, location, location. Scand J Med Sci Sports. 2015;25:34–40. https://doi.org/10.1111/sms.12599.
Mikkelsen KH, Seifert T, Secher NH, et al. Systemic, cerebral and skeletal muscle ketone body and energy metabolism during acute hyper-D-β-hydroxybutyratemia in post-absorptive healthy males. J Clin Endocrinol Metab. 2015;100:636–43. https://doi.org/10.1210/jc.2014-2608.
Olson MS, Dennis SC, DeBuysere MS, Padma A. The regulation of pyruvate dehydrogenase in the isolated perfused rat heart. J Biol Chem. 1978;253:7369–75.
Ashour B, Hansford RG. Effect of fatty acids and ketone on the activity of pyruvate dehydrogenase in skeletal-muscle mitochondria. Biochem J. 1983;214:725–36.
Sato K, Kashiwaya Y, Keon CA, et al. Insulin, ketone bodies, and mitochondrial energy transduction. FASEB J. 1995;9:651–8.
Burgess SC, Iizuka K, Jeoung NH, et al. Carbohydrate-response element-binding protein deletion alters substrate utilization producing an energy-deficient liver. J Biol Chem. 2008;283:1670–8. https://doi.org/10.1074/jbc.M706540200.
Murray AJ, Knight NS, Cole MA, et al. Novel ketone diet enhances physical and cognitive performance. FASEB J. 2016;30:4021–32. https://doi.org/10.1096/fj.201600773R.
Evans M, McSwiney FT, Brady AJ, Egan B. No benefit of ingestion of a ketone monoester supplement on 10-km running performance. Med Sci Sport Exerc. 2019;51:2506–15. https://doi.org/10.1249/MSS.0000000000002065.
Zinn C, Wood M, Williden M, et al. Ketogenic diet benefits body composition and well-being but not performance in a pilot case study of New Zealand endurance athletes. J Int Soc Sports Nutr. 2017;14:22. https://doi.org/10.1186/s12970-017-0180-0.
Maunder E, Kilding AE, Plews DJ. Substrate metabolism during ironman triathlon: different horses on the same courses. Sports Med. 2018;48:2219–26. https://doi.org/10.1007/s40279-018-0938-9.
Tiller NB, Roberts JD, Beasley L, et al. International Society of Sports Nutrition Position Stand: nutritional considerations for single-stage ultra-marathon training and racing. J Int Soc Sports Nutr. 2019;16:1–23. https://doi.org/10.1186/s12970-019-0312-9.
Stellingwerff T, Boon H, Gijsen AP, et al. Carbohydrate supplementation during prolonged cycling exercise spares muscle glycogen but does not affect intramyocellular lipid use. Pflugers Arch. 2007;454:635–47. https://doi.org/10.1007/s00424-007-0236-0.
Stellingwerff T, Boon H, Jonkers RAM, et al. Significant intramyocellular lipid use during prolonged cycling in endurance-trained males as assessed by three different methodologies. Am J Physiol Endocrinol Metab. 2007;292:E1715–23. https://doi.org/10.1152/ajpendo.00678.2006.
van Loon LJC, Koopman R, Stegen JHCH, et al. Intramyocellular lipids form an important substrate source during moderate intensity exercise in endurance-trained males in a fasted state. J Physiol. 2004;553:611–25. https://doi.org/10.1113/jphysiol.2003.052431.
De Bock K, Richter EA, Russell AP, et al. Exercise in the fasted state facilitates fibre type-specific intramyocellular lipid breakdown and stimulates glycogen resynthesis in humans. J Physiol. 2005;564:649–60. https://doi.org/10.1113/jphysiol.2005.083170.
Frayn KN. Fat as a fuel: emerging understanding of the adipose tissue-skeletal muscle axis. Acta Physiol (Oxf). 2010;199:509–18. https://doi.org/10.1111/j.1748-1716.2010.02128.x.
Chrzanowski-Smith OJ, Piatrikova E, Betts JA, et al. Variability in exercise physiology: can capturing intra-individual variation help better understand true inter-individual responses? Eur J Sport Sci. 2019. https://doi.org/10.1080/17461391.2019.1655100.
Helge JW, Watt PW, Richter EA, et al. Fat utilization during exercise: adaptation to a fat-rich diet increases utilization of plasma fatty acids and very low density lipoprotein-triacylglycerol in humans. J Physiol. 2001;537:1009–20.
Braakhuis AJ, Hopkins WG. Impact of dietary antioxidants on sport performance: a review. Sports Med. 2015;45:939–55. https://doi.org/10.1007/s40279-015-0323-x.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No sources of funding were used to assist in the preparation of this article.
Conflict of Interest
Shaw DM, Merien F, Braakhuis A, Maunder E, and Dulson D declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Shaw, D.M., Merien, F., Braakhuis, A. et al. Exogenous Ketone Supplementation and Keto-Adaptation for Endurance Performance: Disentangling the Effects of Two Distinct Metabolic States. Sports Med 50, 641–656 (2020). https://doi.org/10.1007/s40279-019-01246-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40279-019-01246-y