Abstract
Background
The mechanisms responsible for the associations between very preterm birth and a higher risk of poor cardiovascular and metabolic health in adult life are unknown.
Methods
Here, we compare the clinical and molecular phenotypes of healthy, normal-weight young adults (18–27 years), born very preterm (<33 weeks gestational age (GA)) and at full-term (37–42 weeks GA). Outcomes included whole-body MRI, hepatic and muscle 1H MRS, blood pressure measurements and telomere length.
Results
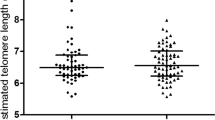
We recruited 156 volunteers, 69 preterm (45 women; 24 men) and 87 born at full-term (45 women; 42 men). Preterm individuals had a significantly altered blood pressure profile, including higher systolic blood pressure (SBP mmHg: preterm men 133.4 ± 10.1, term men 23.0 ± 6.9; preterm women 124.3 ± 7.1, term women 118.4 ± 8.0, p < 0.01 for all). Furthermore, preterm men had fewer long telomeres (145–48.5 kb: preterm men 14.1 ± 0.9%, term men 17.8 ± 1.1%, p < 0.05; 48.5–8.6 kb: preterm men 28.2 ± 2.6, term men 37.0 ± 2.4%, p < 0.001) and a higher proportion of shorter telomeres (4.2–1.3 kb: preterm men 40.4 ± 3.5%, term men 29.9 ± 3.2%, p < 0.01).
Conclusion
Our data indicate that healthy young adults born very preterm manifest clinical and molecular evidence of accelerated ageing.
Similar content being viewed by others
Introduction
Rates of preterm birth and survival are rising globally.1 In high-income countries around 1–2% of births are at or below 33 weeks gestational age (GA) with the majority of these infants surviving to discharge from neonatal care.2 In addition to the well-recognised risks to neurodevelopmental attainment, a growing volume of epidemiological data indicates that preterm birth is a risk to metabolic health in later life. Studies describe higher blood pressure, and increased risk of stroke, metabolic syndrome, type-2 diabetes, and premature death in comparison with birth at full-term.3,4,5 However, current knowledge has been insufficient for translation into preventive health care for this growing population.
We have shown previously that both preterm infants at term6 and adults born preterm7 have an altered body composition, with significantly greater internal-abdominal adipose tissue (IAAT) and intra-hepatocellular lipid (IHCL). Abdominal adipose tissue and ectopic lipid play a major role in the pathogenesis of the metabolic syndrome and correlate strongly with hypertension, insulin resistance and cardiovascular disease,8,9 conditions that typically increase in prevalence with ageing.
In this proof-of-concept cohort study, we postulated that disruption of the normal pattern of third trimester development by very preterm birth would affect multiple systems that influence metabolic health. We tested the primary hypothesis that young non-obese adults born at or below 33 weeks gestation would have greater IAAT than those born at full-term. We also compared ectopic lipid in liver and muscle, biochemical parameters, blood pressure, and serum and urine metabolomes. We sought evidence of a molecular correlate of ageing by evaluating peripheral mononuclear blood cell (PMBC) telomere length.
Methods
We obtained approval for the study from the National Research Ethics Service (London City and East Ethics Committee; REC: 12/LO/1053) and written informed consent from all participants. With the help of Bliss, the national UK preterm and sick baby charity, and the chief investigators of preterm observational cohorts,10,11 we invited the participation of healthy, young adult volunteers. Bliss advertised the study on their website, and the chief investigators sent letters of invitation to individuals from their cohorts for whom they had a current address. The cohorts were the EPICure study of children born below 26 weeks gestation and a follow-up study of extremely preterm children cared for at University College London Hospitals that has been underway since 1979.12 Inclusion criteria were age 18–27 years, with a body mass index (BMI) ≤ 25 kg/m2, born very preterm (≤33 GA) or full-term (37–42 weeks GA). Exclusions were type-2 diabetes, personal or family history of dyslipidaemia, claustrophobia, moderate or severe neurodisability, presence of implanted metallic devices, possibility of pregnancy and any condition requiring medication. Following an overnight fast, participants had a single half-day visit to the Section of Neonatal Medicine, Imperial College London, Chelsea and Westminster Hospital, London, UK. Anthropometric measurements, blood sampling, blood pressure measurements, magnetic resonance imaging (MRI), and magnetic resonance spectroscopy (MRS) were performed. BMI (kg/m2) was calculated from height and weight.
Physical activity was quantified using continuous scores derived from the short form of the IPAQ physical activity questionnaire.13 Standard clinical biochemistry analysis was performed on 8 ml blood samples. Insulin sensitivity was assessed using the Homeostasis Model Assessment of Insulin Resistance (HOMA-IR)12 and Quantitative Insulin Sensitivity Check Index (QUICKI).14 Participants’ post-codes were used to obtain the index of multiple deprivation (IMD) (https://www.gov.uk/government/statistics/english-indices-of-deprivation-2015).15 An automatic digital sphygmomanometer was used to record systolic (SBP) or diastolic (DBP) blood pressure as an average of three readings taken at 1-min intervals, following 5-min rest in a seated position. Participants were instructed on how to wear an ambulatory blood pressure monitor (Spacelabs Medical, Hertfordshire, UK) for 24 h following their study visit. The monitor was programmed to take a reading every 30 min during the day (8 a.m.−10 p.m.) and a reading every hour overnight (10 p.m.−8 a.m.). Data upload, extraction and analysis were carried out blinded to study group. Parameters obtained were ambulatory SBP (aSBP), DBP (aDBP), mean blood pressure (aMBP), pulse pressure (aPP) and heart rate (aHR). Blood pressure monitor outcomes are presented as an overall average or “daytime” and “night-time” average readings. The point at which “night-time” readings began was identified by the expected nocturnal fall in aSBP.16 This was checked against prospectively reported wake/sleep cycles in the study visit questionnaire. In cases where no clear drop was observed, reported wake/sleep cycles were used. When there was a disparity between the observed blood pressure fall and reported sleep time, the blood pressure fall, which has been reported to be more accurate17 was used. “Non-dipping” was defined as a less than 10% fall in nocturnal aSBP. The difference between SBP or DBP readings and their corresponding daytime average ambulatory measurements (daytime average aSBP, daytime average aDBP) was calculated in order to assess a potential white-coat hypertensive response.18
Adipose tissue content and distribution
Whole-body MRI was employed to determine total and regional adipose tissue content, as previously described.19 MR data were acquired on a Siemens Magnetom Avanto 1.5T scanner (Siemens Medical Systems, Erlangen, Germany). Participants were scanned using a T1 weighted fast spin echo sequence; repetition time (TR): 516 ms, echo time (TE) 8.8 ms, echo train length (ETL) 4, three signal averages and using the integrated body coil. Images were acquired with a 10 mm slice thickness, 10 mm gap, a field of view (FOV) of 500 × 500mm, matrix of 512 × 512. Adipose tissue images were analysed independently of the investigators, blinded to subject and group identity, by VardisGroup (London, UK, www.vardisgroup.com), using an image segmentation program (sliceOmatic, Tomovision, Montreal, Canada).20
Intra-hepatocellular and intra-myocellular lipid
For IHCL, 1H MR Spectra were acquired from the right lobe of the liver using a phased-array surface coil and a spine array coil. Single voxel measurements were acquired using 5 STEAM sequences (TE:20, 30, 40, 50 and 60 ms; TR: 1500 ms acquired during breath-hold of 15 s; voxel size: 20 × 20 × 20 mm, with 6 averages. Single voxel measurements were acquired using a PRESS sequence; TR: 3000 ms, TE: 135 ms, 128 averages and 15 × 20 × 20 mm FOV. Water-suppressed and non-water-suppressed spectra were acquired for each muscle. MRS data were analysed using the Advanced Magnetic Resonance (AMARES) fitting algorithm within the Java-based MR user interface (jMRUI) software package, version 5.21 To calculate IMCL, peak areas for water and six lipid resonances were obtained using prior knowledge for the water-suppressed and non-water-suppressed spectra, and T2 corrections. IMCL was determined relative to total muscle water signal and converted to an absolute concentration presented as mmol/kg wet weight (mmol/kg ww).22
Telomere analysis
Telomere length was quantified, as described previously.23 PMBC were extracted from a whole blood sample by density gradient centrifugation. DNA was transferred to nylon membranes by Southern blotting and telomere length measured using Telo TAGGG telomere length assays (Roche Diagnostics, Germany). Telomere signals were analysed using Adobe Photoshop (Adobe Systems, San Jose, CA, USA) and MacBas software (Fujifilm UK, Bedford, UK). Four telomere molecular length groups (145–45.8 kb, 48.5–8.6 kb, 8.6–4.2 kb, 4.2–1.3 kb) were assessed.
Metabolic profiling of urine samples
Samples were analysed using a combination of NMR spectroscopy and global UPLC profiling methods for lipids and polar molecules using previously published methods.24,25,26 Data variables were normalised by a probabilistic quotient method.27 Multivariate data analysis was performed using the SIMCA package (v.13.0.2, Umetrics, Umeå, Sweden). Multivariate Pareto scaled data were modelled using principal components analysis and orthogonal partial least squares discriminant analysis (OPLS-DA). Orthogonal projection to latent structure discriminant analysis (O-PLS-DA)28 was used to optimally model class differences and evaluated using a permutation test.29 Comparisons were made between preterm and term (men and women combined), and separately for the two sexes. The OPLS-DA parameters were evaluated to assess the robustness of each model. A two-tailed t test was applied to the discriminatory features of each model.
Sample size
We approximated the sample size for key outcomes by simulations on pilot data with 10,000 permutations based on a linear model with effects for sex, BMI, preterm birth and a residual error. We determined that a total sample size of 150 participants would have over 80% power (5% significance) to detect a difference of 0.4 l in IAAT, a difference of 4.6 mmHg in SBP and 3.5 mmHg in DBP.
Statistical analysis
We calculated the mean (SD) for each variable and each of the four groups, preterm-women, term-women, preterm-men, term-men. We used multiple linear regression to examine group (preterm−term) and sex (men−women) differences and sex-group interactions, adjusting for age, sex, BMI, IMD and IPAQ score. Significance was taken as p < 0.01 following Bonferroni correction for multiple testing. Unless otherwise stated, comparisons are presented as mean difference with 95% confidence interval (CI). S-IMCL and T-IMCL are presented as geometric mean and ratio (95% CI). To address their non-normally distributed nature, IHCL values were log transformed (0.00001 + x) and a square-root transformation was used for IMCL. Telomere data were analysed using two-way ANOVA with sex and GA as the independent variables and Duncan’s post-hoc testing using Statistica 7 software (Statsoft Inc, Milton Keynes, Buckinghamshire, UK). Values provided are mean difference (95% CI) unless otherwise stated.
Results
We recruited 156 volunteers; 69 born preterm (45 women; 24 men), at a mean GA of 29 weeks (range 23−33) and mean birth weight of 1.26 kg (range 0.51−2.95), and 87 born at full-term (45 women; 42 men). Participants identified themselves predominantly as “white” ethnicity (preterm: 46; full-term: 49), with the remainder spanning a diverse range of single and mixed-race groupings. Preterm participants were significantly shorter (mean difference (95% CI) −5cm (−8 to −2), p < 0.001) but there were no statistically significant differences in weight, BMI or IPAQ score. Baseline characteristics are shown in Table 1. There were no statistically significant differences in AT compartments, total AT, IHCL, S-IMCL or T-IMCL (Table 2).
Compared to full-term born participants, the preterm group had higher mean SBP (6.5 mmHg (4.2−8.9), p < 0.01) and greater differences between daytime single and ambulatory systolic (7.0 mmHg (4.4−9.6), p < 0.01) blood pressure (Table 3). There was a statistically significant preterm-sex interaction for daytime average aSBP, daytime average aPP and overall aPP (all p < 0.01, Table 3). Preterm men showed higher SBP (9.5 mmHg (5.5, 13.6), p < 0.01) daytime aSBP (6.0 mmHg (1.9−10.1), p < 0.01), daytime aPP (greater by 5.9 mmHg (3.2−8.6); p < 0.01) and overall aPP (greater by 5.9 mmHg (3.3−8.5); p < 0.05) (Table 3).
In comparison with term women, preterm women showed lower overall aSBP (−3.2 mmHg (−6.2 to −0.2); p < 0.05) and aMAP (−3.0 mmHg (−5.5 to −0.6); p < 0.05). No significant differences in the proportion of subjects not showing nocturnal dipping were observed between term or preterm groups (p = NS) (proportion of subjects not showing nocturnal dipping: Preterm women: 22% (10/45), Term women: 22% (9/41), Preterm men: 21% (5/24), Term men: 21% (9/42)). Serum leptin was significantly higher in the preterm group (0.28 ng/ml (0.03 to 0.54), p < 0.01) (Supplementary Table 1).
The percentage distribution of each telomere length range is shown in Fig. 1. We identified a significant redistribution of telomere length in preterm men, with fewer long and more short PMBC telomeres compared to full-term men (145–48.5 kb: preterm men 14.1 ± 0.9%; full-term men 17.8 ± 1.1%, p < 0.05; 48.5–8.6 kb: preterm men 28.2 ± 2.6%, full-term men: 37.0 ± 2.4%, p < 0.001); 4.2–1.3 kb: preterm men 40.4 ± 3.5%, full-term men: 29.9 ± 3.2%, p < 0.01). No significant difference in the distribution of telomeres was observed between preterm and full-term women.
The metabolic profiles of full-term compared with preterm participants were not significantly different when modelled either as a whole dataset or stratified according to sex. The profiles were based on multiple metabolite groups including lipids, amines, sugars, phenols, acylcarnitines and organic acids. Similarly, when the profiles were regressed against telomere length, no significant associations were detected.
Discussion
We show that healthy, normal-weight, young adults born very preterm manifest characteristics indicative of greater risk to metabolic and cardiovascular health, compared with those born at full-term. These features, namely higher blood pressure, typically manifest with ageing. We also identified a molecular marker of cellular ageing, a greater proportion of shorter telomeres in preterm men compared to term men. This indicates that the early onset of age-related conditions identified in preterm infants in epidemiological studies is more likely to be a true relationship than a reflection of confounding by factors such as socio-economic disadvantage.30 The lack of significant association of metabolites with term/preterm status indicates that preterm birth had no functional metabolic effect at this age in keeping with their outwardly healthy condition.
The strengths of our study lie in replicating the higher blood pressure we noted in an earlier, smaller study7 and in identifying a molecular correlate in telomere shortening. Participants were young and healthy, and neither overweight nor obese, and the comparisons remained robust to adjustment for potential confounders. Here, a statistically significant increase in IAAT in preterm compared to term men (p < 0.05), as observed in this previous study,7 was lost following adjustment for multiple comparisons.
We analysed telomere length by evaluating the distribution of length categories. This provides more biological insight than assessing mean length, as for a cell to become senescent the telomere length of one chromosome needs to become critically short (<5 kb). Therefore, knowing the proportion of telomeres that are critically short is essential for robust inferences to be made about cellular ageing. The principal weakness is the smaller number of preterm men in relation to women that we were able to recruit. Phenotypic characteristics indicative of risk to metabolic health differ across racial groups and a further limitation is that participants were racially heterogeneous.
Telomeres are hexameric TTAGGG repeats found at the ends of chromosomes responsible for maintenance of chromosomal integrity. They are considered to be robust markers of cellular ageing and senescence in somatic cells and are associated with longevity.31 A novel finding of our study is that, at the age studied, only the men experienced any effect of gestational age at birth upon telomere length with more short and fewer long telomeres in the preterm men compared with the full-term group. Oestrogens are protective against telomere shortening32 which may offer an explanation of why young women did not appear affected.
Epidemiological studies have long suggested increased risk of the metabolic syndrome, and type-2 diabetes in adults born preterm.4 Preterm boys have higher mortality than girls and are generally more susceptible to adverse health outcomes.5 Our data provide further evidence of greater male vulnerability. IAAT is a depot associated with type 2 diabetes, dyslipidaemia and hypertension.33 Whilst not statistically significant, the magnitude of increase in IAAT we see in preterm men, namely by 350 cm3 on average, equivalent to about 290 g AT, is comparable to the significant increase of 510 cm3 seen in our previous study.7 Kuk et al. estimated that 370 g excess IAAT is associated with an 80% increase in 5-year mortality.34 A novel observation is that we identified higher circulating leptin in the preterm cohort, although total AT was similar in the groups. Leptin correlates highly with total AT, so this may indicate leptin resistance, also a feature of ageing.35 We previously identified increased IHCL and soleus IMCL in preterm-born young men,7 markers of ectopic fat deposition that are predictors of peripheral insulin resistance, a key component of the metabolic syndrome,9 but were unable to replicate this observation in the present study.
Higher blood pressure in children and adults born preterm has been reported in several studies from around the world, as has a linear relationship with degree of prematurity.36 In a systematic review and meta-analysis of 27 studies, including over 300,000 participants, we previously identified significantly higher SBP, DBP and aSBP in preterm compared with full-term adults.4 Ambulatory monitoring is considered a more reliable approach to assessing blood pressure, as it is less affected by the anxiety response that accompanies single measurements.37 The clinical relevance of these observations is that every 2 mmHg rise in SBP is associated with a 7% increase in mortality from ischaemic heart disease and a 10% increased risk of stroke.38 Birth before 32 weeks GA is also associated with a near doubling in risk of cerebrovascular disease compared to birth at term and higher mortality from cerebrovascular disease, particularly occlusive stroke.39 Blood pressure measurements in preterm women were inconclusive, with seemingly lower aSBP and aMAP than term women. Preterm women also showed greater differences between single and ambulatory SBP than term women, indicative of a greater stress response as previously reported.40 This suggests that the higher blood pressure in preterm women reported in previous studies may have been driven by the so-called “white-coat” stress response. We recommend that future studies use ambulatory rather than single measurements of blood pressure.
Our data add to a growing recognition that preterm birth disrupts multiple organ systems and leaves a life-long legacy. Our study not only provides evidence for the direct consequences of prematurity, but also insights into the mechanisms by which this occurs. When considered in combination with other epidemiological and experimental data, our study has implications for families, the growing population of young people born preterm who are now reaching adulthood, clinicians, charities, policy-makers, and researchers. Families and in due course the young people themselves should be made aware that very preterm birth is a risk factor for early onset of features indicating susceptibility to cardio-metabolic disease, and should receive general health and lifestyle advice to mitigate against these risks, in particular the benefits of a healthy diet, exercise, and avoidance of smoking. Clinicians should recognise the relevance of asking about preterm birth when taking a general medical history. In our view, existing evidence warrants at the very least, opportunistic measurement of blood pressure in all individuals born preterm. Charities may wish to utilise the findings of this study to improve public understanding of preterm birth, strengthen advocacy and raise funds for research. Preterm birth is closely associated with poor maternal health and socio-economic disadvantage and growing numbers of very preterm survivors are being added to the total population pool. There is also therefore need for policy-makers around the world to direct attention to the wider societal determinants of preterm birth. The challenge for researchers is to determine if the characteristics we identify reflect preterm birth or neonatal care practices, identify causal biological mechanisms, and test candidate intrauterine and new-born interventions to reduce risks to life-long health.
References
Saigal, S. & Doyle, L. W. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet 371, 261–269 (2008). 19.
Sardesai, S., Biniwale, M., Wertheimer, F., Garingo, A. & Ramanathan, R. Evolution of surfactant therapy for respiratory distress syndrome: past, present, and future. Pediatr. Res. 81, 240–248 (2017).
de Jong, F., Monuteaux, M. C., van Elburg, R. M., Gillman, M. W. & Belfort, M. B. Systematic review and meta-analysis of preterm birth and later systolic blood pressure. Hypertension 59, 226–234 (2011).
Parkinson, J. R., Hyde, M. J., Gale, C., Santhakumaran, S. & Modi, N. Preterm birth and the metabolic syndrome in adult life: a systematic review and meta-analysis. Pediatrics 131, e1240–e1263 (2013).
Crump, C., Sundquist, K., Sundquist, J. & Winkleby, M. A. Gestational age at birth and mortality in young adulthood. Jama 306, 1233–1240 (2011).
Uthaya, S. et al. Altered adiposity after extremely preterm birth. Pediatr. Res. 57, 211–215 (2005).
Thomas, E. L. et al. Aberrant adiposity and ectopic lipid deposition characterize the adult phenotype of the preterm infant. Pediatr. Res. 70, 507–512 (2011).
Despres, J. P. et al. Abdominal obesity and the metabolic syndrome: contribution to global cardiometabolic risk. Arterioscler. Thromb. Vasc. Biol. 28, 1039–1049 (2008).
Fabbrini, E. et al. Intrahepatic fat, not visceral fat, is linked with metabolic complications of obesity. Proc. Natl Acad. Sci. USA 106, 15430–15435 (2009).
Wood, N. S. et al. The EPICure study: growth and associated problems in children born at 25 weeks of gestational age or less. Arch. Dis. Child Fetal Neonatal Ed. 88, F492–F500 (2003).
Riley, K., Roth, S., Sellwood, M. & Wyatt, J. S. Survival and neurodevelopmental morbidity at 1 year of age following extremely preterm delivery over a 20-year period: a single centre cohort study. Acta Paediatr. 97, 159–165 (2008).
Matthews, D. R. et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28, 412–419 (1985).
Littlejohns, T. J., Sudlow, C., Allen, N. E. & Collins, R. UK Biobank: opportunities for cardiovascular research. Eur. Heart J. 40, 1158–1166 (2017).
Katz, A. et al. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J. Clin. Endocrinol. Metab. 85, 2402–2410 (2000).
Odgers, C. L., Caspi, A., Bates, C. J., Sampson, R. J. & Moffitt, T. E. Systematic social observation of children’s neighborhoods using Google Street View: a reliable and cost-effective method. J. Child Psychol. Psychiatry, Allied Discip. 53, 1009–1017 (2012).
Bayrakci, U. S., Schaefer, F., Duzova, A., Yigit, S. & Bakkaloglu, A. Abnormal circadian blood pressure regulation in children born preterm. J. Pediatr. 151, 399–403 (2007).
Delaney, A., Pellizzari, M., Speiser, P. W. & Frank, G. R. Pitfalls in the measurement of the nocturnal blood pressure dip in adolescents with type 1 diabetes. Diabetes Care 32, 165–168 (2009).
Martin, C. A. & McGrath, B. P. White-coat hypertension. Clin. Exp. Pharmacol. Physiol. 41, 22–29 (2014).
Thomas, E. L. et al. Hepatic triglyceride content and its relation to body adiposity: a magnetic resonance imaging and proton magnetic resonance spectroscopy study. Gut 54, 122–127 (2005).
Harrington, T. A., Thomas, E. L., Frost, G., Modi, N. & Bell, J. D. Distribution of adipose tissue in the newborn. Pediatr. Res. 55, 437–441 (2004).
van den Boogaart, A., Howe, F. A., Rodrigues, L. M., Stubbs, M. & Griffiths, J. R. In vivo 31P MRS: absolute concentrations, signal-to-noise and prior knowledge. NMR Biomed. 8, 87–93 (1995).
Weis, J., Johansson, L., Ortiz-Nieto, F. & Ahlstrom, H. Assessment of lipids in skeletal muscle by LCModel and AMARES. J. Magn. Reson. Imaging 30, 1124–1129 (2009).
Tarry-Adkins, J. L. et al. Poor maternal nutrition followed by accelerated postnatal growth leads to telomere shortening and increased markers of cell senescence in rat islets. FASEB J. 23, 1521–1528 (2009).
Dona, A. C. et al. Precision high-throughput proton NMR spectroscopy of human urine, serum, and plasma for large-scale metabolic phenotyping. Anal. Chem. 86, 9887–9894 (2014).
Lewis, M. R. et al. Development and application of ultra-performance liquid chromatography-TOF MS for precision large scale urinary metabolic Phenotyping. Anal. Chem. 88, 9004–9013 (2016).
Vorkas, P. A. et al. Untargeted UPLC-MS profiling pipeline to expand tissue metabolome coverage: application to cardiovascular disease. Anal. Chem. 87, 4184–4193 (2015).
Dieterle, F., Ross, A., Schlotterbeck, G. & Senn, H. Probabilistic quotient normalization as robust method to account for dilution of complex biological mixtures. Application in 1H NMR metabonomics. Anal. Chem. 78, 4281–4290 (2006).
Cloarec, O. et al. Evaluation of the orthogonal projection on latent structure model limitations caused by chemical shift variability and improved visualization of biomarker changes in 1H NMR spectroscopic metabonomic studies. Anal. Chem. 77, 517–526 (2005).
Neuhauser, M. & Manly, B. F. The Fisher-Pitman permutation test when testing for differences in mean and variance. Psychol. Rep. 94, 189–194 (2004).
Mathiasen, R., Hansen, B. M., Nybo Anderson, A. M. & Greisen, G. Socio-economic achievements of individuals born very preterm at the age of 27 to 29 years: a nationwide cohort study. Dev. Med. Child Neurol. 51, 901–908 (2009).
Heidinger, B. J. et al. Telomere length in early life predicts lifespan. Proc. Natl Acad. Sci. USA 109, 1743–1748 (2012).
Moller, P. et al. Sex-related differences in length and erosion dynamics of human telomeres favor females. Aging 1, 733–739 (2009).
Despres, J. P. & Lemieux, I. Abdominal obesity and metabolic syndrome. Nature 444, 881–887 (2006).
Kuk, J. L. et al. Visceral fat is an independent predictor of all-cause mortality in men. Obesity (Silver Spring) 14, 336–341 (2006).
Balasko, M., Soos, S., Szekely, M. & Petervari, E. Leptin and aging: Review and questions with particular emphasis on its role in the central regulation of energy balance. J. Chem. Neuroanat. 61–62, 248–255 (2014).
Johansson, S. et al. Risk of high blood pressure among young men increases with the degree of immaturity at birth. Circulation 112, 3430–3436 (2005).
Kamarck, T. W. et al. Psychosocial demands and ambulatory blood pressure: a field assessment approach. Physiol. Behav. 77, 699–704 (2002).
Ritchie, L. D., Campbell, N. C. & Murchie, P. New NICE guidelines for hypertension. BMJ 343, d5644 (2011).
Kajantie, E., Osmond, C. & Eriksson, J. G. Coronary heart disease and stroke in adults born preterm—the Helsinki Birth Cohort Study. Paediatr. Perinat. Epidemiol. 29, 515–519 (2015).
Feldt, K. et al. Cardiovascular reactivity to psychological stressors in late adulthood is predicted by gestational age at birth. J. Hum. Hypertens. 21, 401–410 (2007).
Acknowledgements
We are grateful to Professor John Wyatt and Professor Neil Marlow for assistance in recruiting volunteers. This work was supported by the British Heart Foundation (PG/13/49/30307, PG/09/037/27387, FS/09/029/27902) and the MRC (MC UU12012/04).
Author information
Authors and Affiliations
Contributions
J.R.C.P. contributed to study design, data acquisition, analysis and interpretation, wrote, and revised the final manuscript and has given final approval of this version to be published. R.E. contributed to data acquisition, revised the article and has given final approval of this version to be published. J.L.T.A. contributed to data acquisition, revised the article and has given final approval of this version to be published. N.L. contributed to data analysis, revised the article and has given final approval of this version to be published. S.E.O. contributed to study design, data analysis and interpretation, revised the article and has given final approval of this version to be published. E.H. contributed to study design, data analysis and interpretation, revised the article and has given final approval of this version to be published. N.M. contributed to study design, data analysis and interpretation, and revised the final manuscript and has given final approval of this version to be published. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Parkinson, J.R.C., Emsley, R., Adkins, J.L.T. et al. Clinical and molecular evidence of accelerated ageing following very preterm birth. Pediatr Res 87, 1005–1010 (2020). https://doi.org/10.1038/s41390-019-0709-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-019-0709-9
This article is cited by
-
From premature birth to premature kidney disease: does accelerated aging play a role?
Pediatric Nephrology (2023)
-
A feasibility randomized controlled trial of a NICU rehabilitation program for very low birth weight infants
Scientific Reports (2022)
-
Sex differences in the intergenerational inheritance of metabolic traits
Nature Metabolism (2022)
-
The future of perinatal research
European Journal of Pediatrics (2022)