Abstract
The decarburization behaviour of 60Si2MnA in atmospheres containing 0–21% O2, < 20 ppm–17%H2O, and with or without 8%CO2, at 700–1000 °C, was investigated. The new findings of the current study were: (a) severe decarburization was associated with the formation of wüstite (FeO) scale on the steel surface, (b) the carbon activity at the steel–FeO interface was most likely determined by the reaction equilibrium between FeO and dissolved carbon in steel, (c) when a ferrite layer was able to form, the decarburization tendency was determined by the relative carbon permeability (defined as the product of carbon concentration difference at the two interfaces of the ferrite layer and carbon diffusivity) through the ferrite layer, and therefore, (d) the decarburization tendency at 800 °C was greater than those at 700 and 900 °C as the relative carbon permeability at 800 °C was the greatest. If FeO was absent when heating in dry O2-containing gases, however, possibly as a result of the formation of a SiO2 layer at the steel surface, decarburization was very much alleviated or avoided. At 1000 °C, the decarburization tendency was alleviated even when FeO was able to form because formation of a ferrite layer was not possible and carbon diffusivity in austenite was much lower than that in ferrite. A preformed oxide scale was effective in providing decarburization protection only when the steel was exposed to dry O2-containing atmospheres.













Similar content being viewed by others
References
Chinese National Standard, GB/T 1222-2016: Spring Steels, 2016.
A29/A29M-05, “Standard specification for steel bars, carbon and alloy, hot-wrought, general requirement for”, ASTM International 2006.
BS EN 10089:2002, Hot rolled steels for quenched and tempered springs – Technical delivery conditions, BSi British Standards, 2002.
A. S. Kenneford and G. C. Ellis, Journal of Iron and Steel Institute164, 265 (1950).
M. Assefpour-Dezfuly and A. Brownrigg, Metallurgical Transactions A20A, 1951 (1989).
A. Brownrigg and T. Sritharan, Material Forum10, 58 (1987).
M. J. Gildersleeve, Materials Science and Technology7, 307 (1991).
M. Nomura, H. Morimoto and M. Toyama, ISIJ International40, 619 (2000).
D. Li, D. Anghelina, D. Burzic, J. Zamberger, R. Kienreich, H. Schifferi, W. Krieger and E. Kozeschnik, Steel Research International80, 298 (2009).
D. Li, D. Anghelina, D. Burzic, W. Krieger and E. Kozeschnik, Steel Research International80, 304 (2009).
C. Zhang, Y. Liu, L. Zhou, C. Jiang and J. Xiao, International Journal of Minerals, Metallurgy and Materials19, 116 (2012).
C. Zhang, L. Zhou and Y. Liu, International Journal of Minerals, Metallurgy and Materials20, 720 (2013).
S. Choi and S. Zwaag, ISIJ International52, 549 (2012).
S. Choi and Y. Lee, ISIJ International54, 1682 (2014).
X. Shi, L. Zhao, W. Wang, B. Zeng, L. Zhao, Y. Shan, M. Shen and K. Yang, Transactions of Materials and Heat Treatment34(7), 47 (2013) (in Chinese).
Y. Liu, W. Zhang, Q. Tong and L. Wang, ISIJ International54, 1920 (2014).
F. Zhao, C. Zhang, Q. Xiu, Y. Tan, S. Zhang and Y. Liu, Materials Science Forum817, 132 (2015).
Y. Liu, W. Zhang, Q. Tong and Q. Sun, International Journal of Iron and Steel Research23, 1316 (2016).
F. Zhao, C. L. Zhang and Y. Z. Liu, Archives of Metallurgy and Materials61, 1715 (2016).
W. A. Pennington, Transactions of the American Society for Metals37, 48 (1946).
N. Birks, Decarburization, The Iron and Steel Institute Publication 133, London, 1969, pp. 1.
N. Birks and W. Jackson, Journal Iron and Steel Institute208, 81 (1970).
N. Birks, G. H. Meier and F. S. Pettit, Introduction to the High-Temperature Oxidation of Metals, First Edition, Edward Arnold, London, 1983, pp 175; Second Edition, Cambridge University Press, Cambridge, UK, 2006, pp. 151–162.
L. S. Darken, Transactions of the Metallurgical Society of AIME180, 430 (1949); H. K. D. H. Bhadeshia, Metallurgical and Materials Transactions A41A, 1605 (2010).
R. Y. Chen, Oxidation of Metals89, 1 (2018).
W. E. Jominy and D. W. Murphy, Transaction of the American Society for Steel Treating18, 19 (1930).
A. Rahmel and J. Tobolski, Werkstoffe und Korrosion16, 662 (1965).
A. Rahmel, Werkstoffe und Korrosion16, 837 (1965).
M. Fukumoto, S. Maeda, S. Hayashi and T. Narita, Tetsu-to-Hagané86, 526 (2000).
A. A. Mouayd, A. Koltsov, E. Sutter and B. Tribollet, Materials Chemistry and Physics143, 996 (2014).
J. Baud, A. Ferrier, J. Manenc and J. Bénard, Oxidation of Metals9, 69 (1975).
K. Sachs, Decarburization, The Iron and Steel Institute Publication 133, London, 1969, pp. 13.
R. Beaumont, Decarburization, The Iron and Steel Institute Publication 133, London, 1969, pp. 34.
E 1077 – 01, “Standard Test Methods for Estimating the Depth of Decarburization of Steel Specimens”, ASTM International, PA, United States, 2001.
G. F. Vander Voort, Advanced Materials & Processes, 173(2), 22 (2015).
Australian Standard®, “Carbon and low alloy steel – measurement of decarburization”, AS 2003 – 1991, Reconfirmed 2016, Standards Australia, NSW, Australia
O. Kubaschewski and C. B. Alcock, Metallurgical Thermochemistry, Fifth ed, (Pergamon Press, Oxford, 1979), pp. 378–384.
W. Cao, S. -L. Chen, F. Zhang, K. Wu, Y. Yang, Y.A. Chang, R. Schmid-Fetzer and W. A. Oates, CALPHAD: Computer Coupling of Phase Diagrams and Thermochemistry33, 328 (2009).
PanFe, Thermodynamic database for Fe-based alloys, CompuTherm, LLC: Middleton WI 53562, USA, 2019.
Y. R. Chen, Y. Liu and X. Xu, Oxidation of 60Si2MnA steel in atmospheres containing different levels of oxygen, water vapour and carbon dioxide at 700–1000 °C, Oxidation of Metals, 2019. https://doi.org/10.1007/s11085-019-09944-8.
A. D. Pelton, P. Koukkari, R. Pajarre and G. Eriksson, Journal Chemical Thermodynamics72, 16 (2014).
M. Hillert, Phase Equilibria, Phase Diagrams and Phase Transformations – Their Thermodynamic Basis, 2nd ed, (Cambridge University Press, Cambridge UK, 2008), pp. 311–315.
F. D. Richardson and J. H. E. Jeffes, Journal of Iron Steel Institute160, 261 (1948).
J. A. Lobo and G. H. Gaiger, Metallurgical Transactions A.7A, 1347 (1976).
T. Ellis, I. M. Davidson and C. Bodsworth, Journal of Iron Steel Institute201, 582 (1963).
M. A. Krishtal, Diffusion Processes in Iron Alloys, Israel Program for Scientific Translation, Jerusalem, 1970.
Y. R. Chen, F. Zhang and Y. Liu, Unpublished results submitted to Metallurgical and Materials Transactions A, October 2019.
G. Parrish and G. S. Harper, Production Gas Carburizing, (Pergamon Press, Oxford, 1985), p. 116.
W. C. Leslie, The Physical Metallurgy of Steels, (Hemisphere Publishing Corporation, Washington and McGraw-Hill Book Company, New York, 1981).
A. Atkinson, Corrosion Science22, 87 (1982).
R. Collin, S. Gunnarson and D. Thulin, Journal of Iron Steel Institute210, 785 (1972).
T. Wada, H. Wada, J. F. Elliott and J. Chipman, Metallurgical Transactions3, 1657 (1972).
D. R. Poirier, Transactions of the Metallurgical Society of AIME242, 685 (1968).
R. P. Smith, Transactions of the Metallurgical Society of AIME224, 105 (1962).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix 1: Determination of the Relative Carbon Permeability Through the Ferrite Layer [25]
Appendix 1: Determination of the Relative Carbon Permeability Through the Ferrite Layer [25]
In determining the equilibrium carbon composition at the FeO–steel interface, it was assumed that the dissolved carbon in steel, [C], could react with FeO via the following reactions:
and the overall reaction of these reactions was:
Using the published data [37, 43], the standard Gibbs free energy of formation of Reaction (17) was given by
When Reaction (17) reached equilibrium,
where R is the gas constant (R = 8.314 J mol−1 K−1) and T temperature in K.
The \( \frac{{P_{\text{CO}} }}{{P_{{{\text{CO}}_{2} }} }} \) thus obtained was used to determine the equilibrium carbon activity at the interface assuming \( P_{\text{CO}} \) + \( P_{{{\text{CO}}_{2} }} \) = 1 atm from the following reaction:
The standard Gibbs free energy of formation of Reaction (20) was given by [37, 43],
where \( a_{c} \) was the equilibrium activity of carbon at the scale–steel interface with graphite being the standard state. From Eq. (21), we obtained,
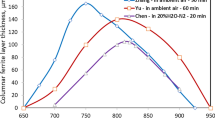
The calculated \( a_{\text{c}} \) as a function of temperature is plotted in Fig. 14.
After the equilibrium carbon activity at the interface was determined, the corresponding carbon concentration in the steel at the FeO–steel interface could be calculated using the known relationships between carbon activity and carbon composition. For dissolved carbon in α-Fe, the relationship given by Lobo and Gaiger [44] to express the activity coefficient of carbon in ferrite for carbon steel, \( \varUpsilon_{\text{C}} \left( {\text{ferrite}} \right) \), was used,
where \( X_{\text{C}} \) was the equilibrium mole fraction of carbon in ferrite.
If the steel phase in equilibrium with the scale was γ-Fe, there were several equations available in the literature [45, 51,52,53] to relate the carbon activity to steel carbon composition. In this study, the equation given by Ellis et al. [45] was used:
Using Eqs. (22) to (24), the equilibrium carbon concentrations at the FeO–steel interface, depending on which steel phase was stable, was calculated.
When a ferrite layer formed on the steel surface, the difference in the carbon concentration between two interfaces of the ferrite layer, \( \Delta C_{{C\;{\text{in}}\;\alpha }} \), provided a driving force for carbon diffusion through the ferrite layer. When the ferrite layer was thin, the carbon distribution in it could be approximated as having a linear composition gradient and the carbon diffusion flux through this layer could be described using the simplified Fick’s first law:
where \( J_{\text{C}}^{{\alpha - {\text{Fe}}}} \) = diffusion flux of carbon through the ferrite layer in mol cm−2 s−1 (moles per square centimetre per second); \( D_{\text{C}}^{{\alpha - {\text{Fe}}}} \) = diffusion coefficient of carbon in ferrite, in cm2 s−1; \( C_{{C\;{\text{in}}\;\alpha }}^{\alpha /\gamma } \) = carbon composition on the ferrite side at the BCC–FCC interface in mol cm−3; \( C_{{C\;{\text{in}}\;\alpha }}^{{\alpha /{\text{FeO}}}} \) = carbon composition in ferrite at the BCC–FeO interface in mol cm−3; \( X \) = thickness of the ferrite layer, in cm.
From Eq. (25), it was seen that for a certain thickness of the decarburization layer, \( X \), the rate of carbon diffusion was determined by the product of carbon diffusivity \( D_{\text{C}}^{{\alpha - {\text{Fe}}}} \) and the carbon composition difference between the two interfaces of the ferrite layer, \( \Delta C_{{C\;{\text{in}}\;\alpha }} = C_{{C\;{\text{in}}\;\alpha }}^{\alpha /\gamma } - C_{{C\;{\text{in}}\;\alpha }}^{{\alpha /{\text{FeO}}}} \). Following the approach used by Smith [54], the following product was defined as the relative permeability (\( P_{\text{C}}^{{\alpha - {\text{Fe}}}} \) in mol cm−1 s−1) of carbon through the ferrite layer,
Substitution of Eq. (26) in Eq. (25) yielded
From Eq. (27), it was seen that a greater relative permeability immediately led to a greater carbon flux for a given ferrite layer thickness.
Rights and permissions
About this article
Cite this article
Chen, Y.R., Xu, X. & Liu, Y. Decarburization of 60Si2MnA in Atmospheres Containing Different Levels of Oxygen, Water Vapour and Carbon Dioxide at 700–1000 °C. Oxid Met 93, 105–129 (2020). https://doi.org/10.1007/s11085-019-09949-3
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11085-019-09949-3