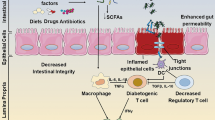
Abstract
Diseases intrinsic to the pancreas such as pancreatitis, pancreatic cancer and type 1 diabetes mellitus impart substantial health and financial burdens on society but identification of novel mechanisms contributing to these pathologies are slow to emerge. A novel area of research suggests that pancreatic-specific disorders might be modulated by the gut microbiota, either through a local (direct pancreatic influence) or in a remote (nonpancreatic) fashion. In this Perspectives, we examine literature implicating microorganisms in diseases of the pancreas, specifically pancreatitis, type 1 diabetes mellitus and pancreatic ductal adenocarcinoma. We also discuss evidence of an inherent pancreatic microbiota and the influence of the intestinal microbiota as it relates to disease association and development. In doing so, we address pitfalls in the current literature and areas of investigation that are needed to advance a developing field of research that has clinical potential to reduce the societal burden of pancreatic diseases.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Gale, E. A. M. The rise of childhood type 1 diabetes in the 20th century. Diabetes 51, 3353–3361 (2002).
Petrov, M. S. & Yadav, D. Global epidemiology and holistic prevention of pancreatitis. Nat. Rev. Gastroenterol. Hepatol. 16, 175–184 (2018).
American Cancer Society. Cancer Facts & Figures 2019. American Cancer Society https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2019/cancer-facts-and-figures-2019.pdf (2019).
Adeloye, D. et al. Global and regional estimates of the morbidity due to type I diabetes among children aged 0-4 years: a systematic review and analysis. J. Glob. Health 8, 021101 (2018).
Karjula, H. et al. Long-term outcome and causes of death for working-age patients hospitalized due to acute pancreatitis with a median follow-up of 10 years. Ann. Surg. 269, 932–936 (2019).
American Diabetes Association. Economic costs of diabetes in the U.S. in 2017. Diabetes Care 41, 917–928 (2018).
Willey, V. J. et al. Estimating the real-world cost of diabetes mellitus in the United States during an 8-year period using 2 cost methodologies. Am. Health Drug. Benefits 11, 310–318 (2018).
Peery, A. F. et al. Burden and cost of gastrointestinal, liver, and pancreatic diseases in the United States: update 2018. Gastroenterology 156, 254–272.e11 (2019).
Thomas, R. M. et al. Intestinal microbiota enhances pancreatic carcinogenesis in preclinical models. Carcinogenesis 39, 1068–1078 (2018).
Pushalkar, S. et al. The pancreatic cancer microbiome promotes oncogenesis by induction of innate and adaptive immune suppression. Cancer Discov. 8, 403–416 (2018).
Sun, J. et al. Pancreatic β-cells limit autoimmune diabetes via an immunoregulatory antimicrobial peptide expressed under the influence of the gut microbiota. Immunity 43, 304–317 (2015).
Ahuja, M. et al. Orai1-mediated antimicrobial secretion from pancreatic acini shapes the gut microbiome and regulates gut innate immunity. Cell Metab. 25, 635–646 (2017).
Geller, L. T. et al. Potential role of intratumor bacteria in mediating tumor resistance to the chemotherapeutic drug gemcitabine. Science 357, 1156–1160 (2017).
Kahn, S. E., Hull, R. L. & Utzschneider, K. M. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 444, 840–846 (2006).
Tilg, H. & Moschen, A. R. Microbiota and diabetes: an evolving relationship. Gut 63, 1513–1521 (2014).
Tilg, H., Zmora, N., Adolph, T. E. & Elinav, E. The intestinal microbiota fuelling metabolic inflammation. Nat. Rev. Immunol. https://doi.org/10.1038/s41577-019-0198-4 (2019).
Sharma, S. & Tripathi, P. Gut microbiome and type 2 diabetes: where we are and where to go? J. Nutr. Biochem. 63, 101–108 (2019).
Marietta, E., Horwath, I., Balakrishnan, B. & Taneja, V. Role of the intestinal microbiome in autoimmune diseases and its use in treatments. Cell Immunol. 339, 50–58 (2018).
Desai, M. S. et al. A dietary fiber-deprived gut microbiota degrades the colonic mucus barrier and enhances pathogen susceptibility. Cell 167, 1339–1353.e21 (2016).
Shepherd, E. S., DeLoache, W. C., Pruss, K. M., Whitaker, W. R. & Sonnenburg, J. L. An exclusive metabolic niche enables strain engraftment in the gut microbiota. Nature 557, 434–438 (2018).
Sethi, V. et al. Gut microbiota promotes tumor growth in mice by modulating immune response. Gastroenterology 155, 33–37.e6 (2018).
de Goffau, M. C. et al. Human placenta has no microbiome but can contain potential pathogens. Nature 572, 329–334 (2019).
Bawagan, J. Babies get critical gut bacteria from their mother at birth, not from placenta, study suggests. Science https://doi.org/10.1126/science.aay9546 (2019).
Bevins, C. L. & Salzman, N. H. Paneth cells, antimicrobial peptides and maintenance of intestinal homeostasis. Nat. Rev. Microbiol. 9, 356–368 (2011).
Medveczky, P., Szmola, R. & Sahin-Tóth, M. Proteolytic activation of human pancreatitis-associated protein is required for peptidoglycan binding and bacterial aggregation. Biochem. J. 420, 335–343 (2009).
Doyle, C. J. et al. The proteome of normal pancreatic juice. Pancreas 41, 186–194 (2012).
Stenwall, A., Ingvast, S., Skog, O. & Korsgren, O. Characterization of host defense molecules in the human pancreas. Islets 11, 89–101 (2019).
Hogan, P. G. The STIM1-ORAI1 microdomain. Cell Calcium 58, 357–367 (2015).
Fan, X. et al. Human oral microbiome and prospective risk for pancreatic cancer: a population-based nested case-control study. Gut 67, 120–127 (2018).
Farrell, J. J. et al. Variations of oral microbiota are associated with pancreatic diseases including pancreatic cancer. Gut 61, 582–588 (2012).
Del Castillo, E. et al. The microbiomes of pancreatic and duodenum tissue overlap and are highly subject specific but differ between pancreatic cancer and noncancer subjects. Cancer Epidemiol. Biomarkers Prev. 28, 370–383 (2019).
Diehl, G. E. et al. Microbiota restricts trafficking of bacteria to mesenteric lymph nodes by CX3CR1hi cells. Nature 494, 116–120 (2013).
Bravo-Blas, A. et al. Salmonella enterica serovar typhimurium travels to mesenteric lymph nodes both with host cells and autonomously. J. Immunol. 202, 260–267 (2019).
Varol, C. et al. Intestinal lamina propria dendritic cell subsets have different origin and functions. Immunity 31, 502–512 (2009).
Widdison, A. L., Karanjia, N. D. & Reber, H. A. Routes of spread of pathogens into the pancreas in a feline model of acute pancreatitis. Gut 35, 1306–1310 (1994).
Banks, P. A. et al. Classification of acute pancreatitis-2012: revision of the Atlanta classification and definitions by international consensus. Gut 62, 102–111 (2013).
Lee, P. J. & Papachristou, G. I. New insights into acute pancreatitis. Nat. Rev. Gastroenterol. Hepatol. 16, 479–496 (2019).
Koziel, D., Gluszek, S., Kowalik, A., Chlopek, M. & Pieciak, L. Genetic mutations in SPINK1, CFTR, CTRC genes in acute pancreatitis. BMC Gastroenterol. 15, 70 (2015).
Yadav, D., O’Connell, M. & Papachristou, G. I. Natural history following the first attack of acute pancreatitis. Am. J. Gastroenterol. 107, 1096–1103 (2012).
Spicak, J. et al. Alcoholic chronic pancreatitis and liver cirrhosis: coincidence and differences in lifestyle. Pancreatology 12, 311–316 (2012).
Veena, A. B., Rajesh, G., Varghese, J., Sundaram, K. R. & Balakrishnan, V. Alcoholic chronic pancreatitis and alcoholic liver cirrhosis: differences in alcohol use habits and patterns in Indian subjects. Pancreas 41, 703–706 (2012).
Tu, J. et al. Endocrine and exocrine pancreatic insufficiency after acute pancreatitis: long-term follow-up study. BMC Gastroenterol. 17, 114 (2017).
Ahmed Ali, U. et al. Risk of recurrent pancreatitis and progression to chronic pancreatitis after a first episode of acute pancreatitis. Clin. Gastroenterol. Hepatol. 14, 738–746 (2016).
Beger, H. G., Bittner, R., Block, S. & Büchler, M. Bacterial contamination of pancreatic necrosis. A prospective clinical study. Gastroenterology 91, 433–438 (1986).
Büchler, M. W. et al. Acute necrotizing pancreatitis: treatment strategy according to the status of infection. Ann. Surg. 232, 619–626 (2000).
Isenmann, R. et al. Prophylactic antibiotic treatment in patients with predicted severe acute pancreatitis: a placebo-controlled, double-blind trial. Gastroenterology 126, 997–1004 (2004).
Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature 486, 207–214 (2012).
Grice, E. A. et al. Topographical and temporal diversity of the human skin microbiome. Science 324, 1190–1192 (2009).
Gilbert, J. A. et al. Current understanding of the human microbiome. Nat. Med. 24, 392–400 (2018).
O’Boyle, C. J. et al. Microbiology of bacterial translocation in humans. Gut 42, 29–35 (1998).
MacFie, J. et al. Gut origin of sepsis: a prospective study investigating associations between bacterial translocation, gastric microflora, and septic morbidity. Gut 45, 223–228 (1999).
Ammori, B. J. et al. Early increase in intestinal permeability in patients with severe acute pancreatitis: correlation with endotoxemia, organ failure, and mortality. J. Gastrointest. Surg. 3, 252–262 (1999).
Sonika, U. et al. Mechanism of increased intestinal permeability in acute pancreatitis: alteration in tight junction proteins. J. Clin. Gastroenterol. 51, 461–466 (2017).
Liu, H., Li, W., Wang, X., Li, J. & Yu, W. Early gut mucosal dysfunction in patients with acute pancreatitis. Pancreas 36, 192–196 (2008).
Zhang, X. M. et al. Intestinal microbial community differs between acute pancreatitis patients and healthy volunteers. Biomed. Environ. Sci. 31, 81–86 (2018).
Lankisch, P. G. et al. Natural history of acute pancreatitis: a long-term population-based study. Am. J. Gastroenterol. 104, 2797–2805; quiz 2806 (2009).
Jandhyala, S. M. et al. Altered intestinal microbiota in patients with chronic pancreatitis: implications in diabetes and metabolic abnormalities. Sci. Rep. 7, 43640 (2017).
Uchida, K., Masamune, A., Shimosegawa, T. & Okazaki, K. Prevalence of IgG4-related disease in Japan based on nationwide survey in 2009. Int. J. Rheumatol. 2012, 358371 (2012).
Hart, P. A., Zen, Y. & Chari, S. T. Recent advances in autoimmune pancreatitis. Gastroenterology 149, 39–51 (2015).
Madhani, K. & Farrell, J. J. Autoimmune pancreatitis: an update on diagnosis and management. Gastroenterol. Clin. North. Am. 45, 29–43 (2016).
Hamada, S., Masamune, A., Nabeshima, T. & Shimosegawa, T. Differences in gut microbiota profiles between autoimmune pancreatitis and chronic pancreatitis. Tohoku J. Exp. Med. 244, 113–117 (2018).
Isaiah, A., Parambeth, J. C., Steiner, J. M., Lidbury, J. A. & Suchodolski, J. S. The fecal microbiome of dogs with exocrine pancreatic insufficiency. Anaerobe 45, 50–58 (2017).
Nishiyama, H. et al. Supplementation of pancreatic digestive enzymes alters the composition of intestinal microbiota in mice. Biochem. Biophys. Res. Commun. 495, 273–279 (2018).
Sabbah, E. et al. Genetic, autoimmune, and clinical characteristics of childhood- and adult-onset type 1 diabetes. Diabetes Care 23, 1326–1332 (2000).
Patterson, C. C. et al. Incidence trends for childhood type 1 diabetes in Europe during 1989-2003 and predicted new cases 2005-20: a multicentre prospective registration study. Lancet 373, 2027–2033 (2009).
Cooper, G. S. & Stroehla, B. C. The epidemiology of autoimmune diseases. Autoimmun. Rev. 2, 119–125 (2003).
Klein, N. P. et al. Rates of autoimmune diseases in Kaiser Permanente for use in vaccine adverse event safety studies. Vaccine 28, 1062–1068 (2010).
Hooper, L. V., Littman, D. R. & Macpherson, A. J. Interactions between the microbiota and the immune system. Science 336, 1268–1273 (2012).
Hollister, E. B., Gao, C. & Versalovic, J. Compositional and functional features of the gastrointestinal microbiome and their effects on human health. Gastroenterology 146, 1449–1458 (2014).
de Goffau, M. C. et al. Aberrant gut microbiota composition at the onset of type 1 diabetes in young children. Diabetologia 57, 1569–1577 (2014).
de Goffau, M. C. et al. Fecal microbiota composition differs between children with β-cell autoimmunity and those without. Diabetes 62, 1238–1244 (2013).
Vatanen, T. et al. The human gut microbiome in early-onset type 1 diabetes from the TEDDY study. Nature 562, 589–594 (2018).
Mejía-León, M. E., Petrosino, J. F., Ajami, N. J., Domínguez-Bello, M. G. & de la Barca, A. M. C. Fecal microbiota imbalance in Mexican children with type 1 diabetes. Sci. Rep. 4, 3814 (2014).
Davis-Richardson, A. G. et al. Bacteroides dorei dominates gut microbiome prior to autoimmunity in Finnish children at high risk for type 1 diabetes. Front. Microbiol. 5, 678 (2014).
Brown, C. T. et al. Gut microbiome metagenomics analysis suggests a functional model for the development of autoimmunity for type 1 diabetes. PLOS ONE 6, e25792 (2011).
Greiner, T. U., Hyötyläinen, T., Knip, M., Bäckhed, F. & Orešič, M. The gut microbiota modulates glycaemic control and serum metabolite profiles in non-obese diabetic mice. PLOS ONE 9, e110359 (2014).
Akira, S. & Takeda, K. Toll-like receptor signalling. Nat. Rev. Immunol. 4, 499–511 (2004).
Takeda, K. & Akira, S. TLR signaling pathways. Semin. Immunol. 16, 3–9 (2004).
Takeuchi, O. & Akira, S. Pattern recognition receptors and inflammation. Cell 140, 805–820 (2010).
Wen, L. et al. Innate immunity and intestinal microbiota in the development of type 1 diabetes. Nature 455, 1109–1113 (2008).
Dewhirst, F. E. et al. Phylogeny of the defined murine microbiota: altered Schaedler flora. Appl. Environ. Microbiol. 65, 3287–3292 (1999).
Compagno, M. et al. Mutations of multiple genes cause deregulation of NF-kappaB in diffuse large B-cell lymphoma. Nature 459, 717–721 (2009).
Yamamoto, M. et al. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science 301, 640–643 (2003).
Burrows, M. P., Volchkov, P., Kobayashi, K. S. & Chervonsky, A. V. Microbiota regulates type 1 diabetes through Toll-like receptors. Proc. Natl Acad. Sci. USA 112, 9973–9977 (2015).
Bray, F. et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 68, 394–424 (2018).
Rahib, L. et al. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 74, 2913–2921 (2014).
Rawla, P., Sunkara, T. & Gaduputi, V. Epidemiology of pancreatic cancer: global trends, etiology and risk factors. World J. Oncol. 10, 10–27 (2019).
Michaud, D. S. et al. Plasma antibodies to oral bacteria and risk of pancreatic cancer in a large European prospective cohort study. Gut 62, 1764–1770 (2013).
Ren, Z. et al. Gut microbial profile analysis by MiSeq sequencing of pancreatic carcinoma patients in China. Oncotarget 8, 95176–95191 (2017).
Stolzenberg-Solomon, R. Z. et al. Tooth loss, pancreatic cancer, and Helicobacter pylori. Am. J. Clin. Nutr. 78, 176–181 (2003).
Hujoel, P. P., Drangsholt, M., Spiekerman, C. & Weiss, N. S. An exploration of the periodontitis-cancer association. Ann. Epidemiol. 13, 312–316 (2003).
Ahn, J., Segers, S. & Hayes, R. B. Periodontal disease, porphyromonas gingivalis serum antibody levels and orodigestive cancer mortality. Carcinogenesis 33, 1055–1058 (2012).
Gaiser, R. A. et al. Enrichment of oral microbiota in early cystic precursors to invasive pancreatic cancer. Gut 68, 2186–2194 (2019).
Matthaei, H. et al. miRNA biomarkers in cyst fluid augment the diagnosis and management of pancreatic cysts. Clin. Cancer Res. 18, 4713–4724 (2012).
Thomas, R. M. & Fleming, J. B. MicroRNA dissects out dangerous pancreatic cysts from all the rest. Clin. Cancer Res. 18, 4482–4484 (2012).
Hata, T. et al. Predicting the grade of dysplasia of pancreatic cystic neoplasms using cyst fluid DNA methylation markers. Clin. Cancer Res. 23, 3935–3944 (2017).
Tanaka, M. et al. Revisions of international consensus Fukuoka guidelines for the management of IPMN of the pancreas. Pancreatology 17, 738–753 (2017).
Zaura, E., Keijser, B. J. F., Huse, S. M. & Crielaard, W. Defining the healthy “core microbiome” of oral microbial communities. BMC Microbiol. 9, 259 (2009).
Ochoa-Repáraz, J., Mielcarz, D. W., Begum-Haque, S. & Kasper, L. H. Gut, bugs, and brain: role of commensal bacteria in the control of central nervous system disease. Ann. Neurol. 69, 240–247 (2011).
Schwabe, R. F. & Jobin, C. The microbiome and cancer. Nat. Rev. Cancer 13, 800–812 (2013).
Sears, C. L. & Garrett, W. S. Microbes, microbiota, and colon cancer. Cell Host Microbe 15, 317–328 (2014).
Tang, W. H. W. & Hazen, S. L. The contributory role of gut microbiota in cardiovascular disease. J. Clin. Invest. 124, 4204–4211 (2014).
Tomkovich, S. et al. Locoregional effects of microbiota in a preclinical model of colon carcinogenesis. Cancer Res. 77, 2620–2632 (2017).
Dejea, C. M. et al. Patients with familial adenomatous polyposis harbor colonic biofilms containing tumorigenic bacteria. Science 359, 592–597 (2018).
Saad, A. M., Turk, T., Al-Husseini, M. J. & Abdel-Rahman, O. Trends in pancreatic adenocarcinoma incidence and mortality in the United States in the last four decades; a SEER-based study. BMC Cancer 18, 688 (2018).
Meng, Z. et al. Tumor location as an indicator of survival in T1 resectable pancreatic ductal adenocarcinoma: a propensity score-matched analysis. BMC Gastroenterol. 19, 59 (2019).
Winer, L. K. et al. The impact of tumor location on resection and survival for pancreatic ductal adenocarcinoma. J. Surg. Res. 239, 60–66 (2019).
Ou, G. et al. Proximal small intestinal microbiota and identification of rod-shaped bacteria associated with childhood celiac disease. Am. J. Gastroenterol. 104, 3058–3067 (2009).
Nistal, E. et al. Differences of small intestinal bacteria populations in adults and children with/without celiac disease: effect of age, gluten diet, and disease. Inflamm. Bowel Dis. 18, 649–656 (2012).
Riquelme, E. et al. Tumor microbiome diversity and composition influence pancreatic cancer outcomes. Cell 178, 795–806.e12 (2019).
Kojima, T. et al. Tight junctions in human pancreatic duct epithelial cells. Tissue Barriers 1, e24894 (2013).
Li, J. et al. Fungi in gastrointestinal tracts of human and mice: from community to functions. Microb. Ecol. 75, 821–829 (2018).
Aykut, B. et al. The fungal mycobiome promotes pancreatic oncogenesis via activation of MBL. Nature 574, 264–267 (2019).
Hingorani, S. R. et al. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell 4, 437–450 (2003).
Hingorani, S. R. et al. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell 7, 469–483 (2005).
Burris, H. A. et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J. Clin. Oncol. 15, 2403–2413 (1997).
Conroy, T. et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N. Engl. J. Med. 364, 1817–1825 (2011).
Grindey, G. B., Hertel, L. W. & Plunkett, W. Cytotoxicity and antitumor activity of 2’,2’-difluorodeoxycytidine (gemcitabine). Cancer Invest. 8, 313 (1990).
Roberts, A. B., Wallace, B. D., Venkatesh, M. K., Mani, S. & Redinbo, M. R. Molecular insights into microbial β-glucuronidase inhibition to abrogate CPT-11 toxicity. Mol. Pharmacol. 84, 208–217 (2013).
Wallace, B. D. et al. Structure and inhibition of microbiome β-glucuronidases essential to the alleviation of cancer drug toxicity. Chem. Biol. 22, 1238–1249 (2015).
Gopalakrishnan, V. et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science 359, 97–103 (2018).
Matson, V. et al. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science 359, 104–108 (2018).
Gharaibeh, R. Z. & Jobin, C. Microbiota and cancer immunotherapy: in search of microbial signals. Gut 68, 385–388 (2018).
Routy, B. et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 359, 91–97 (2018).
McQuade, J. L., Daniel, C. R., Helmink, B. A. & Wargo, J. A. Modulating the microbiome to improve therapeutic response in cancer. Lancet Oncol. 20, e77–e91 (2019).
Schmitt, M., Klonowski-Stumpe, H., Eckert, M., Lüthen, R. & Häussinger, D. Disruption of paracellular sealing is an early event in acute caerulein-pancreatitis. Pancreas 28, 181–190 (2004).
Martinez, K. B., Leone, V. & Chang, E. B. Microbial metabolites in health and disease: navigating the unknown in search of function. J. Biol. Chem. 292, 8553–8559 (2017).
Postler, T. S. & Ghosh, S. Understanding the holobiont: how microbial metabolites affect human health and shape the immune system. Cell Metab. 26, 110–130 (2017).
Chen, H. et al. A forward chemical genetic screen reveals gut microbiota metabolites that modulate host physiology. Cell 177, 1217–1231.e18 (2019).
Arthur, J. C. et al. Intestinal inflammation targets cancer-inducing activity of the microbiota. Science 338, 120–123 (2012).
Vétizou, M. et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science 350, 1079–1084 (2015).
Sivan, A. et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science 350, 1084–1089 (2015).
Brahmer, J. R. et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N. Engl. J. Med. 366, 2455–2465 (2012).
Le, D. T. et al. Evaluation of ipilimumab in combination with allogeneic pancreatic tumor cells transfected with a GM-CSF gene in previously treated pancreatic cancer. J. Immunother. 36, 382–389 (2013).
Besselink, M. G. et al. Probiotic prophylaxis in predicted severe acute pancreatitis: a randomised, double-blind, placebo-controlled trial. Lancet 371, 651–659 (2008).
Nakatsu, G. et al. Alterations in enteric virome are associated with colorectal cancer and survival outcomes. Gastroenterology 155, 529–541.e5 (2018).
Coker, O. O. et al. Enteric fungal microbiota dysbiosis and ecological alterations in colorectal cancer. Gut 68, 654–662 (2019).
Kostic, A. D. et al. The dynamics of the human infant gut microbiome in development and in progression toward type 1 diabetes. Cell Host Microbe 17, 260–273 (2015).
Endesfelder, D. et al. Compromised gut microbiota networks in children with anti-islet cell autoimmunity. Diabetes 63, 2006–2014 (2014).
Alkanani, A. K. et al. Alterations in intestinal microbiota correlate with susceptibility to type 1 diabetes. Diabetes 64, 3510–3520 (2015).
Hu, Y., Peng, J., Li, F., Wong, F. S. & Wen, L. Evaluation of different mucosal microbiota leads to gut microbiota-based prediction of type 1 diabetes in NOD mice. Sci. Rep. 8, 15451 (2018).
Acknowledgements
C.J. is funded by NIH grants R01DK073338 and R01AT008623, and the University of Florida Department of Medicine Gatorade Fund. R.M.T. is supported by the American Cancer Society Norma and Rich DiMarco Mentored Research Scholar Grant (MRSG-17-228-01-TBG) and University of Florida Health Cancer Center Pilot Project Grant.
Reviewer information
Nature Reviews Gastroenterology & Hepatology thanks J. Diana, D. Saxena and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Thomas, R.M., Jobin, C. Microbiota in pancreatic health and disease: the next frontier in microbiome research. Nat Rev Gastroenterol Hepatol 17, 53–64 (2020). https://doi.org/10.1038/s41575-019-0242-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41575-019-0242-7
This article is cited by
-
Integrative metagenomic and metabolomic analyses reveal the potential of gut microbiota to exacerbate acute pancreatitis
npj Biofilms and Microbiomes (2024)
-
Pancreatitis affects gut microbiota via metabolites and inflammatory cytokines: an exploratory two-step Mendelian randomisation study
Molecular Genetics and Genomics (2024)
-
Causal effect between gut microbiota and pancreatic cancer: a two-sample Mendelian randomization study
BMC Cancer (2023)
-
Pathobionts from chemically disrupted gut microbiota induce insulin-dependent diabetes in mice
Microbiome (2023)
-
Comparison of DNA extraction methods for 16S rRNA gene sequencing in the analysis of the human gut microbiome
Scientific Reports (2023)