Abstract
Background/Objectives
The reported association between maternal antibiotic use and childhood obesity, if true, could change obstetric practice. However, it is unclear whether the reported association was due to antibiotics, or underlying infection or both. To examine the independent contributions of maternal infection and antibiotic use separately, we conducted a birth cohort study among Kaiser Permanente Northern California (KPNC) members.
Subjects/Methods
The study consisted of 145,393 mother-child dyads. The KPNC electronic medical records provided data on maternal infections, antibiotic use during pregnancy, and longitudinal anthropometric measurements throughout childhood. Obesity was defined by BMI using CDC criteria. Mixed effects logistic regression for repeated measurements was used to analyze multiple BMI measurements per child (five measurements per child on average).
Results
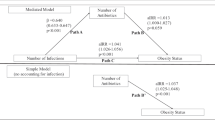
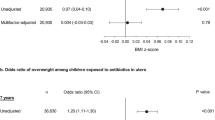
After controlling for confounders using propensity score methodology, there was no increased risk associated with maternal antibiotic use during pregnancy once underlying infection was controlled for: OR = 0.97 (95% CI: 0.92–1.01). There was also no association with timing of use or use of broad-spectrum antibiotics, nor a dose-response relationship. In contrast, maternal untreated infection (without antibiotic use) during pregnancy was associated with a statistically significant risk of childhood obesity compared with mothers without infection: odds ratio (OR) = 1.09 (95% confidence interval (CI): 1.03–1.16). The association was stronger for GBS positive infection (OR = 1.16) than GBS negative infections (OR = 1.08). These results were further confirmed by a discordant sibling study. This discordant sibling study allowed additional control of unmeasured confounders including genetic, maternal intrauterine, and familiar factors. The consistent findings from this sibling study enhances the reproducibility of our findings.
Conclusions
It is maternal infection, NOT antibiotic use, during pregnancy that is associated with increased risk of childhood obesity. While use of antibiotics should always be judicious, in the context of preventing childhood obesity, the focus should be on reducing maternal infections during pregnancy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Skinner AC, Perrin EM, Skelton JA. Prevalence of obesity and severe obesity in US children, 1999–2014. Obesity. 2016;24:1116–23.
Centers for Disease Control and Prevention. Childhood obesity facts. USA: Centers for Disease Control and Prevention; 2015. http://www.cdc.gov/healthyschools/obesity/facts.htm.
World Health Organization. Population-based approaches to childhood obesity prevention. Geneva, Switzerland: World Health Organization; 2012. http://www.who.int/dietphysicalactivity/childhood/WHO_new_childhoodobesity_PREVENTION_27nov_HR_PRINT_OK.pdf.
Institute of Medicine (IOM). Accelerating progress in obesity prevention: solving the weight of the nation. Washington, DC: IOM; 2012.
Mathur R, Barlow GM. Obesity and the microbiome. Expert Rev Gastroenterol Hepatol. 2015;9:1087–99.
Komaroff AL. The microbiome and risk for obesity and diabetes. JAMA. 2017;317:355–6.
Tilg H, Adolph TE. Influence of the human intestinal microbiome on obesity and metabolic dysfunction. Curr Opin Pediatr. 2015;26:496–501.
Fukuda S, Ohno H. Gut microbiome and metabolic diseases. Semin Immunopathol. 2014;36:103–14.
Fanaro S, Chierici R, Guerrini P, Vigi V. Intestinal microflora in early infancy: composition and development. Acta Paediatr. 2003;91:48–55.
Penders J, Thijs C, Vink C, Stelma FF, Snijders B, Kummeling I, et al. Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics. 2006;118:511–21.
Munyaka PM, Khafipour E, Ghia JE. External influence of early childhood establishment of gut microbiota and subsequent health implications. Front Pediatr. 2014;2:109.
Kalliomaki M, Collado MC, Salminen S, Isolauri E. Early differences in fecal microbiota composition in children may predict overweight. Am J Clin Nutr. 2008;87:534–8.
Soderborg TK, Borengasser SJ, Barbour LA, Friedman JE. Microbial transmission from mothers with obesity or diabetes to infants: an innovative opportunity to interrupt a vicious cycle. Diabetologia. 2016;59:895–906.
Rautava S. Microbial composition of the initial colonization of newborns. Nestle Nutr Inst Workshop Series. 2017;88:11–21.
Dunn AB, Jordan S, Baker BJ, Carlson NS. The maternal infant microbiome: considerations for labor and birth. MCN Am J Matern Child Nurs. 2017;42:318–25.
Mueller NT, Whyatt R, Hoepner L, Oberfield S, Dominguez-Bello MG, Widen EM, et al. Prenatal exposure to antibiotics, cesarean section and risk of childhood obesity. Int J Obes. 2015;39:665–70.
Mor A, Antonsen S, Kahlert J, Holsteen V, Jorgensen S, Holm-Pedersen J, et al. Prenatal exposure to systemic antibacterials and overweight and obesity in Danish schoolchildren: a prevalence study. Int J Obes. 2015;39:1450–5.
Cassidy-Bushrow AE, Burmeister C, Havstad S, Levin AM, Lynch SV, Ownby DR, et al. Prenatal antimicrobial use and early-childhood body mass index. Int J Obes. 2018;42:1–7.
Poulsen MN, Pollak J, Bailey-Davis L, Hirsch AG, Glass TA, Schwartz BS. Associations of prenatal and childhood antibiotic use with child body mass index at age 3 years. Obesity. 2017;25:438–44.
Wang B, Liu J, Zhang Y, Yan C, Wang H, Jiang F. et al. Prenatal exposure to antibiotics and risk of childhood obesity in a multi-center cohort study. Am J Epidemiol. 2018;187:2159–2167.
Centers for Disease Control and Prevention. Prevention of perinatal group B streptococcal disease: revised guidelines from CDC, 2010. Atlanta, GA: Centers for Disease Control and Prevention; 2010. https://www.cdc.gov/mmwr/preview/mmwrhtml/rr5910a1.htm.
American College of Obstetricians and Gynecologists Committee on Obstetric Practice. ACOG Committee Opinion No. 485: Prevention of early-onset group B streptococcal disease in newborns. Obstet Gynecol. 2011;117:1019–27.
Dhurandhar NV. A framework for identification of infections that contribute to human obesity. Lancet Infect Dis. 2011;11:963–9.
Deriu E, Boxx GM, He X, Pan C, Benavidez SD, Cen L, et al. Influenza virus affects intestinal microbiota and secondary salmonella infection in the gut through type I interferons. PLoS Pathog. 2016;12:e1005572.
Suprunenko T, Hofer MJ. The emerging role of interferon regulatory factor 9 in the antiviral host response and beyond. Cytokine Growth Factor Rev. 2016;29:35–43.
Li DK, Chen H, Ferber JR, Odouli R. Infection and antibiotic use in infancy and risk of childhood obesity: a longitudinal birth cohort study. Lancet Diab Endocrinol. 2016;5:18–25.
Cocoros NM, Lash TL, Norgaard M, Farkas DK, DeMaria A Jr., Sorensen HT. Hospitalized prenatal and childhood infections and obesity in Danish male conscripts. Annals Epidemiol. 2013;23:307–13.
Gordon NP. Similarity of the adult kaiser permanente membership in northern california to the insured and general population in northern california: statistics from the 2011–12 California Health Interview Survey. Oakland, CA: Kaiser Permanente Division of Research; 2015.
Gordon NP. A comparison of sociodemographic and health characteristics of the kaiser permanente northern california membership derived from two data sources: the 2008 Member Health Survey and the 2007 California Health Interview Survey. Oakland, CA: Kaiser Permanente Division of Research; 2012.
Centers for Disease Control and Prevention. Body mass index: considerations for practitioners. Atlanta, GA: Centers for Disease Control and Prevention; 2011. http://www.cdc.gov/obesity/downloads/BMIforPactitioners.pdf.
Kasai C, Sugimoto K, Moritani I, Tanaka J, Oya Y, Inoue H, et al. Comparison of the gut microbiota composition between obese and non-obese individuals in a Japanese population, as analyzed by terminal restriction fragment length polymorphism and next-generation sequencing. BMC Gastroenterol. 2015;15:100–9.
Korpela K, Zijlmans MA, Kuitunen M, Kukkonen K, Savilahti E, Salonen A, et al. Childhood BMI in relation to microbiota in infancy and lifetime antibiotic use. Microbiome. 2017;5:26.
Angelakis E, Armougom F, Million M, Raoult D. The relationship between gut microbiota and weight gain in humans. Future Microbiol. 2012;7:91–109.
Bailey LC, Forrest CB, Zhang P, Richards TM, Livshits A, DeRusso PA. Association of antibiotics in infancy with early childhood obesity. JAMA Pediatr. 2014;168:1063–9.
Qin N, Zheng B, Yao J, Guo L, Zuo J, Wu L, et al. Influence of H7N9 virus infection and associated treatment on human gut microbiota. Sci Rep. 2015;5:14771.
De Vlaminck I, Khush KK, Strehl C, Kohli B, Luikart H, Neff NF, et al. Temporal response of the human virome to immunosuppression and antiviral therapy. Cell. 2013;155:1178–87.
Oever JT, Netea MG. The bacteriome-mycobiome interaction and antifungal host defense. Eur J Immunol. 2014;44:3182–91.
Hayes KS, Bancroft AJ, Goldrick M, Portsmouth C, Roberts IS, Grencis RK. Exploitation of the intestinal microflora by the parasitic nematode Trichuris muris. Science. 2010;328:1391–4.
Thompson MG, Li DK, Shifflett P, Sokolow LZ, Ferber JR, Kurosky S, et al. Effectiveness of seasonal trivalent influenza vaccine for preventing influenza virus illness among pregnant women: a population-based case-control study during the 2010–2011 and 2011–2012 influenza seasons. Clin Infect Dis. 2014;58:449–57.
Sokolow LZ, Naleway AL, Li DK, Shifflett P, Reynolds S, Henninger ML, et al. Severity of influenza and noninfluenza acute respiratory illness among pregnant women, 2010-2012. Am J Obstet Gynecol. 2015;212:202 e1–11.
Zerbo O, Qian Y, Yoshida C, Grether JK, Van de Water J, Croen LA. Maternal infection during pregnancy and autism spectrum disorders. J Autism Dev Disord. 2015;45:4015–25.
Barlow SE. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics. 2007;120:S164–S92.
Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, et al. 2000 CDC growth charts for the United States: methods and development. Vital Health Stat. 2002;11:1–190.
Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22.
Hubbard AE, Ahern J, Fleischer NL, Van der Laan M, Lippman SA, Jewell N, et al. To GEE or not to GEE: comparing population average and mixed models for estimating the associations between neighborhood risk factors and health. Epidemiology. 2010;21:467–74.
Hosmer DW, Lemeshow S. Logistic regression, 2nd ed. New York: John Wiley and Sons, Inc.; 2000.
Schwartz BS, Pollak J, Bailey-Davis L, Hirsch AG, Cosgrove SE, Nau C, et al. Antibiotic use and childhood body mass index trajectory. Int J Obes. 2016;40:615–21.
Wen X, Kleinman K, Gillman MW, Rifas-Shiman SL, Taveras EM. Childhood body mass index trajectories: modeling, characterizing, pairwise correlations and socio-demographic predictors of trajectory characteristics. BMC Med Res Methodol. 2012;12:38 https://doi.org/10.1186/1471-2288-12-38.:38-12.
Chivers P, Hands B, Parker H, Beilin L, Kendall G, Bulsara M. Longitudinal modelling of body mass index from birth to 14 years. Obes Facts. 2009;2:302–10.
Aker AM, Johns L, McElrath TF, Cantonwine DE, Mukherjee B, Meeker JD. Associations between maternal phenol and paraben urinary biomarkers and maternal hormones during pregnancy: a repeated measures study. Environ Int. 2018;113:341–9.
Glynn RJ, Schneeweiss S, Sturmer T. Indications for propensity scores and review of their use in pharmacoepidemiology. Basic Clin Pharmacol Toxicol. 2006;98:253–9.
Sturmer T, Joshi M, Glynn RJ, Avorn J, Rothman KJ, Schneeweiss S. A review of the application of propensity score methods yielded increasing use, advantages in specific settings, but not substantially different estimates compared with conventional multivariable methods. J Clin Epidemiol. 2006;59:437–47.
Wang J, Li F, Wei H, Lian ZX, Sun R, Tian Z. Respiratory influenza virus infection induces intestinal immune injury via microbiota-mediated Th17 cell-dependent inflammation. J Exp Med. 2014;211:2397–410.
Acknowledgements
This study was funded by The Kaiser Permanente Center for Effectiveness & Safety Research. We also thank Andrew Hirst for his help in data analysis.
Author contributions
D-KL conceived the concept, designed the study, obtained funding, oversaw the data gathering and analyses, and is responsible for the interpretation of results, and drafting and finalizing the manuscript. HC and JF were responsible for data management and analysis, and interpretation of the data. RO was involved in the study management and preparation of the manuscript. D-KL is the guarantor of this paper who took full responsibility for the conduct of the study, had access to the data, and controlled the decision to publish.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Tweet: It is maternal infection, not antibiotic use, during pregnancy that is associated with the risk of childhood obesity.
Rights and permissions
About this article
Cite this article
Li, DK., Chen, H., Ferber, J. et al. Maternal infection and antibiotic use in pregnancy and the risk of childhood obesity in offspring: a birth cohort study. Int J Obes 44, 771–780 (2020). https://doi.org/10.1038/s41366-019-0501-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41366-019-0501-2