Abstract
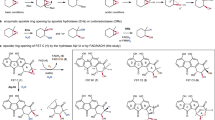
Flavin-dependent ‘ene’-reductases (EREDs) are exquisite catalysts for effecting stereoselective reductions. Although these reactions typically proceed through a hydride transfer mechanism, we recently found that EREDs can also catalyse reductive dehalogenations and cyclizations via single electron transfer mechanisms. Here, we demonstrate that these enzymes can catalyse redox-neutral radical cyclizations to produce enantioenriched oxindoles from α-haloamides. This transformation is a C–C bond-forming reaction currently unknown in nature and one for which there are no catalytic asymmetric examples. Mechanistic studies indicate the reaction proceeds via the flavin semiquinone/quinone redox couple, where ground-state flavin semiquinone provides the electron for substrate reduction and flavin quinone oxidizes the vinylogous α-amido radical formed after cyclization. This mechanistic manifold was previously unknown for this enzyme family, highlighting the versatility of EREDs in asymmetric synthesis.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
Data are available in the Supplementary Information or from the corresponding author upon request.
References
De Meijere, A. & Diederich, F. Metal-catalyzed Cross-coupling Reactions (Wiley-VCH, 2004).
Scheffler, U. & Mahrwald, R. Recent advances in organocatalytic methods for asymmetric C–C bond formation. Chem. Eur. J. 19, 14346–14396 (2013).
Bornscheuer, U. T. et al. Engineering the third wave of biocatalysis. Nature 485, 185–194 (2012).
Yoon, T. P. & Jacobsen, E. N. Privileged chiral catalysts. Science 299, 1691–1693 (2003).
Pandya, C., Farelli, J. D., Dunaway-Mariano, D. & Allen, K. N. Enzyme promiscuity: engine of evolutionary innovation. J. Biol. Chem. 289, 30229–30236 (2014).
Martínez, A. T. et al. Oxidoreductases on their way to industrial biotransformations. Biotechnol. Adv. 35, 815–831 (2017).
Dong, J. et al. Biocatalytic oxidation reactions: a chemist's perspective. Angew. Chem. Int. Ed. 57, 9238–9261 (2018).
Kazlauskas, R. J. & Bornscheuer, U. in Comprehensive Chirality Vol. 7 (eds Carreira, E. M. & Yamamoto H.) 465–480 (Elsevier, 2012).
Bornscheuer, U. T. & Kazlauskas, R. Catalytic promiscuity in biocatalysis: using old enzymes to form new bonds and follow new pathways. Angew. Chem. Int. Ed. 43, 6032–6040 (2004).
Emmanuel, M. E., Greenberg, N. R., Oblinsky, D. G. & Hyster, T. K. Accessing non-natural reactivity by irradiating nicotinamide-dependent ketoreductases with light. Nature 540, 414–417 (2016).
Biegasiewicz, K. F., Cooper, S. J., Emmanuel, M. A. & Miller, D. C. Catalytic promiscuity enabled by photoredox catalysis in nicotinamide-dependent oxidoreductases. Nat. Chem. 10, 770–775 (2018).
Sibi, M. P. & Porter, N. A. Enantioselective free radical reactions. Acc. Chem. Res. 32, 163–171 (1999).
Meggers, E. Asymmetric catalysis activated by visible light. Chem. Commun. 51, 3290–3301 (2015).
Toogood, H. S. & Scrutton, N. S. Discovery, characterization, engineering and application of ene-reductases for industrial biocatalysis. ACS Catal. 8, 3532–3549 (2018).
Winkler, C. K., Faber, K. & Hall, M. Biocatalytic reduction of activated C=C-bonds and beyond: emerging trends. Curr. Opin. Chem. Biol. 43, 97–105 (2018).
Heckenbichler, K. et al. Asymmetric reductive carbocyclization using engineered ene reductases. Angew. Chem. Int. Ed. 57, 7240–7244 (2018).
Miura, R. Versatility and specificity in flavoenzymes: control mechanisms of flavin reactivity. Chem. Rec. 1, 183–194 (2001).
Sandoval, B. A., Meichan, A. J. & Hyster, T. K. Enantioselective hydrogen atom transfer: discovery of catalytic promiscuity in flavin-dependent ‘ene’-reductases. J. Am. Chem. Soc. 139, 11313–11316 (2017).
Biegasiewicz, K. F. et al. Photoexcitation of a flavoenzyme enables a stereocontrolled radical cyclization. Science 364, 1166–1169 (2019).
Shaw, M. H., Twilton, J. & MacMillan, D. W. C. Photoredox catalysis in organic chemistry. J. Org. Chem. 81, 6898–6926 (2016).
Ju, X., Liang, Y., Jia, P., Li, W. & Yu, W. Synthesis of oxindoles via visible light photoredox catalysis. Org. Biomol. Chem. 10, 498–501 (2012).
Zhou, F., Liu, Y.-L. & Zhou, J. Catalytic asymmetric synthesis of oxindoles bearing a tetrasubstituted stereocenter at the C-3 position. Adv. Synth. Catal. 352, 1381–1407 (2010).
Tang, M.-C., Zou, Y., Watanabe, K., Walsh, C. T. & Tang, Y. Oxidative cyclization in natural product biosynthesis. Chem. Rev. 117, 5226–5333 (2017).
Walsh, C. T. & Wencewicz, T. A. Flavoenzymes: versatile catalysts in biosynthetic pathways. Nat. Prod. Rep. 30, 175–200 (2013).
Walsh, C. T. & Tang, Y. Recent advances in enzymatic complexity generations: cyclization reactions. Biochemistry 57, 3087–3104 (2018).
Barna, T. et al. Crystal structure of bacterial morphinone reductase and properties of the C191A mutant enzyme. J. Biol. Chem. 277, 30976–30983 (2002).
Steward, R. C. & Massey, V. Potentiometric studies of native and flavin-substituted old yellow enzyme. J. Biol. Chem. 260, 13639–13647 (1985).
Massey, V., Stankovich, M. & Hemmerich, P. Light-mediated reduction of flavoproteins with flavins as catalysts. Biochemistry 17, 1–8 (1978).
Taglieber, A., Schulz, F., Hollmann, F., Rusek, M. & Reetz, M. T. Light-driven biocatalytic oxidation and reduction reactions: scope and limitations. ChemBioChem 9, 565–572 (2008).
Peers, M. K. et al. Light-driven biocatalytic reduction of α,β-unsaturated compounds by ene reductases employing transition metal complexes as photosensitizers. Catal. Sci. Technol. 6, 169–177 (2016).
Strassner, J., Fürholz, A., Macheroux, P., Amrhein, N. & Schaller, A. A homolog of old yellow enzyme in tomato. Spectral properties and substrate specificity of the recombinant protein. J. Biol. Chem. 274, 35067–35073 (1999).
Murthy, Y. V. S. N. & Massey, V. Synthesis and properties of 8-CN-flavin nucleotide analogs and studies with flavoproteins. J. Biol. Chem. 273, 8975–8982 (1998).
Knight, A. M. et al. Diverse engineered heme proteins enable stereodivergent cyclopropanation of unactivated alkenes. ACS Cent. Sci. 4, 372–377 (2018).
Acknowledgements
T.K.H. acknowledges NIHGMS (R01 GM127703), the Searle Scholar Program (SSP-2017-1741) and the Princeton Catalysis Initiate for Support. B.K. acknowledges the NSF for a Graduate Research Fellowship (DGE-1656466). D.G.O. acknowledges support from the Postgraduate Scholarships Doctoral Program of NSERC. Work by B.K., D.G.O. and G.D.S. was supported BioLEC, an Energy Frontier Research Center funded by DOE, Office of Science, BES under award no. DE-SC0019370.
Author information
Authors and Affiliations
Contributions
T.K.H. conceived and directed the project. T.K.H., M.J.B., A.J.M. and K.F.B. designed the experiments. M.J.B., A.J.M. and K.F.B. performed and analysed the experiments. D.G.O. performed the EPR measurements and B.K. and D.G.O. performed the time-correlated single photon counting) and transient absorption measurements. B.K., D.G.O. and G.D.S. analysed and interpreted the spectroscopy results. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary Information
Supplementary materials and methods, data and Figs. 1–25.
Rights and permissions
About this article
Cite this article
Black, M.J., Biegasiewicz, K.F., Meichan, A.J. et al. Asymmetric redox-neutral radical cyclization catalysed by flavin-dependent ‘ene’-reductases. Nat. Chem. 12, 71–75 (2020). https://doi.org/10.1038/s41557-019-0370-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-019-0370-2
This article is cited by
-
Enzyme-controlled stereoselective radical cyclization to arenes enabled by metalloredox biocatalysis
Nature Catalysis (2023)
-
Stereoselective construction of β-, γ- and δ-lactam rings via enzymatic C–H amidation
Nature Catalysis (2023)
-
Using enzymes to tame nitrogen-centred radicals for enantioselective hydroamination
Nature Chemistry (2023)
-
Enantioselective decarboxylative alkylation using synergistic photoenzymatic catalysis
Nature Catalysis (2023)
-
Bifunctional organic photocatalysts for enantioselective visible-light-mediated photocatalysis
Nature Synthesis (2023)