Abstract
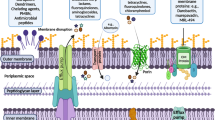
Gram-negative bacteria and their complex cell envelope, which comprises an outer membrane and an inner membrane, are an important and attractive system for studying the translocation of small molecules across biological membranes. In the outer membrane of Enterobacteriaceae, trimeric porins control the cellular uptake of small molecules, including nutrients and antibacterial agents. The relatively slow porin-mediated passive uptake across the outer membrane and active efflux via efflux pumps in the inner membrane creates a permeability barrier. The synergistic action of outer membrane permeability, efflux pump activities and enzymatic degradation efficiently reduces the intracellular concentrations of small molecules and contributes to the emergence of antibiotic resistance. In this Review, we discuss recent advances in our understanding of the molecular and functional roles of general porins in small-molecule translocation in Enterobacteriaceae and consider the crucial contribution of porins in antibiotic resistance.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Nikaido, H. Outer membrane barrier as a mechanism of antimicrobial resistance. Antimicrob. Agents Chemother. 33, 1831–1836 (1989).
Zgurskaya, H. I., Löpez, C. A. & Gnanakaran, S. Permeability barrier of Gram-negative cell envelopes and approaches to bypass it. ACS Infect. Dis. 1, 512–522 (2015).
Hancock, R. E. W. The bacterial outer membrane as a drug barrier. Trends Microbiol. 5, 37–42 (1997).
Nikaido, H. Molecular basis of bacterial outer membrane permeability revisited. Microbiol. Mol. Biol. Rev. 67, 593–656 (2003).
Pagès, J.-M., James, C. E. & Winterhalter, M. The porin and the permeating antibiotic: a selective diffusion barrier in Gram-negative bacteria. Nat. Rev. Microbiol. 6, 893–903 (2008).
Masi, M., Réfrégiers, M., Pos, K. M. & Pagès, J.-M. Mechanisms of envelope permeability and antibiotic influx and efflux in gram-negative bacteria. Nat. Microbiol. 2, 17001 (2017).
Yan, J. J., Wang, M. C., Zheng, P. X., Tsai, L. H. & Wu, J. J. Associations of the major international high-risk resistant clones and virulent clones with specific ompK36 allele groups in Klebsiella pneumoniae in Taiwan. New Microbes New Infect. 5, 1–4 (2015).
Galdiero, S. et al. Microbe-host interactions: structure and role of Gram-negative bacterial porins. Curr. Protein Pept. Sci. 13, 843–854 (2012).
Liu, Y.-F. et al. Loss of outer membrane protein C in Escherichia coli contributes to both antibiotic resistance and escaping antibody-dependent bactericidal activity. Infect. Immun. 80, 1815–1822 (2012).
Salerno-Gonçalves, R. et al. Use of a novel antigen expressing system to study the Salmonella enterica serovar Typhi protein recognition by T cells. PLOS Negl. Trop. Dis. 11, e0005912 (2017).
Pérez-Toledo, M. et al. Salmonella Typhi porins OmpC and OmpF are potent adjuvants for T-dependent and T-independent antigens. Front. Immunol. 8, 230 (2017).
El-Khatib, M. et al. Porin self-association enables cell-to-cell contact in Providencia stuartii floating communities. Proc. Natl Acad. Sci. USA 115, E2220–E2228 (2018).
Cramer, W. A., Sharma, O. & Zakharov, S. D. On mechanisms of colicin import: the outer membrane quandary. Biochem. J. 475, 3903–3915 (2018).
Walsh, C. Antibiotics: Actions, Origins, Resistance (American Society of Microbiology, 2003).
Bryskier, A. (ed.) Antimicrobial Agents: Antibacterials and Antifungals (American Society of Microbiology, 2005).
Dam, S., Pagès, J.-M. & Masi, M. Stress responses, outer membrane permeability control and antimicrobial resistance in Enterobacteriaceae. Microbiology 164, 260–267 (2018).
Bush, K. Past and present perspectives on β-lactamases. Antimicrob. Agents Chemother. 62, e01076–18 (2018).
Weiss, M. S. & Schulz, G. E. Structure of porin refined at 1.8 Å resolution. J. Mol. Biol. 227, 493–509 (1992).
Cowan, S. W. et al. Crystal structures explain functional properties of two E. coli porins. Nature 358, 727 (1992).
Lou, K.-L. et al. Structural and functional characterization of OmpF porin mutants selected for larger pore size. I. Crystallographic analysis. J. Biol. Chem. 271, 20669–20675 (1996).
Phale, P. S. et al. Role of charged residues at the OmpF porin channel constriction probed by mutagenesis and simulation. Biochemistry 40, 6319–6325 (2001).
Baslé, A., Rummel, G., Storici, P., Rosenbusch, J. P. & Schirmer, T. Crystal structure of osmoporin OmpC from E. coli at 2.0 Å. J. Mol. Biol. 362, 933–942 (2006).
Dutzler, R. et al. Crystal structure and functional characterization of OmpK36, the osmoporin of Klebsiella pneumoniae. Structure 7, 425–434 (1999).
Balasubramaniam, D., Arockiasamy, A., Kumar, P. D., Sharma, A. & Krishnaswamy, S. Asymmetric pore occupancy in crystal structure of OmpF porin from Salmonella typhi. J. Struct. Biol. 178, 233–244 (2012).
Acosta-Gutiérrez, S. et al. Getting drugs into Gram-negative bacteria: Rational rules for permeation through general porins. ACS Infect. Dis. 4, 1487–1498 (2018).
Arunmanee, W. et al. Gram-negative trimeric porins have specific LPS binding sites that are essential for porin biogenesis. Proc. Natl Acad. Sci. USA 113, E5034–E5043 (2016).
Malinverni, J. C. & Silhavy, T. J. An ABC transport system that maintains lipid asymmetry in the gram-negative outer membrane. Proc. Natl Acad. Sci. USA 106, 8009–8014 (2009).
Abellón-Ruiz, J. et al. Structural basis for maintenance of bacterial outer membrane lipid asymmetry. Nat. Microbiol. 2, 1616 (2017).
Schulz, G. E. Structure-function relationships in the membrane channel porin as based on a 1.8 Å resolution crystal structure. in Membrane Proteins: Structures, Interactions and Models (eds Pullman, A., Jortner, J. & Pullman, B.) 403–412 (Springer, 1992).
Dhakshnamoorthy, B., Ziervogel, B. K., Blachowicz, L. & Roux, B. A structural study of ion permeation in OmpF porin from anomalous X-ray diffraction and molecular dynamics simulations. J. Am. Chem. Soc. 135, 16561–16568 (2013).
Misra, R. & Benson, S. A. Isolation and characterization of OmpC porin mutants with altered pore properties. J. Bacteriol. 170, 528–533 (1988).
Lou, H. et al. Altered antibiotic transport in OmpC mutants isolated from a series of clinical strains of multi-drug resistant E. Coli. PLOS ONE 6, e25825 (2011).
Kojima, S. & Nikaido, H. Permeation rates of penicillins indicate that Escherichia coli porins function principally as nonspecific channels. Proc. Natl Acad. Sci USA 110, E2629–E2634 (2013).
Nestorovich, E. M., Danelon, C., Winterhalter, M. & Bezrukov, S. M. Designed to penetrate: Time-resolved interaction of single antibiotic molecules with bacterial pores. Proc. Natl Acad. Sci. USA 99, 9789–9794 (2002).
Ziervogel, B. K. & Roux, B. The binding of antibiotics in OmpF porin. Structure 21, 76–87 (2013).
Dutzler, R., Wang, Y.-F., Rizkallah, P. J., Rosenbusch, J. P. & Schirmer, T. Crystal structures of various maltooligosaccharides bound to maltoporin reveal a specific sugar translocation pathway. Structure 4, 127–134 (1996).
Ye, J. & van den Berg, B. Crystal structure of the bacterial nucleoside transporter Tsx. EMBO J. 23, 3187–3195 (2004).
Richter, M. F. et al. Predictive compound accumulation rules yield a broad-spectrum antibiotic. Nature 545, 299–304 (2017).
Sugawara, E., Kojima, S. & Nikaido, H. Klebsiella pneumoniae major porins OmpK35 and OmpK36 allow more efficient diffusion of β-lactams than their Escherichia coli homologs OmpF and OmpC. J Bacteriol. 198, 3200–3208 (2016).
Cai, H., Rose, K., Liang, L.-H., Dunham, S. & Stover, C. Development of a liquid chromatography/mass spectrometry-based drug accumulation assay in Pseudomonas aeruginosa. Anal. Biochem. 385, 321–325 (2009).
Schumacher, A. et al. Intracellular accumulation of linezolid in Escherichia coli, Citrobacter freundii and Enterobacter aerogenes: role of enhanced efflux pump activity and inactivation. J. Antimicrob. Chemother. 59, 1261–1264 (2007).
Davis, T. D., Gerry, ChristopherJ. & Tan, D. S. General platform for systematic quantitative evaluation of small-molecule permeability in bacteria. ACS Chem. Biol. 9, 2535–2544 (2014).
Zhou, Y. et al. Thinking outside the “bug”: A unique assay to measure intracellular drug penetration in Gram-negative bacteria. Anal. Chem. 87, 3579–3584 (2015).
Iyer, R. et al. Evaluating LC-MS/MS to measure accumulation of compounds within bacteria. ACS Infect. Dis. 4, 1336–1345 (2018).
Six, D. A., Krucker, T. & Leeds, J. A. Advances and challenges in bacterial compound accumulation assays for drug discovery. Curr. Opin. Chem. Biol. 44, 9–15 (2018).
Prochnow, H. et al. Subcellular quantification of uptake in Gram-negative bacteria. Anal. Chem. 91, 1863–1872 (2019).
Vergalli, J. et al. Fluoroquinolone structure and translocation flux across bacterial membrane. Sci. Rep. 7, 9821 (2017).
Vergalli, J. et al. Spectrofluorimetric quantification of antibiotic drug concentration in bacterial cells for the characterization of translocation across bacterial membranes. Nat. Protoc. 13, 1348–1361 (2018).
Allam, A. et al. Microspectrofluorimetry to dissect the permeation of ceftazidime in Gram-negative bacteria. Sci. Rep. 7, 986 (2017).
Andersen, C., Jordy, M. & Benz, R. Evaluation of the rate constants of sugar transport through maltoporin (LamB) of Escherichia coli from the sugar-induced current noise. J. Gen. Physiol. 105, 385–401 (1995).
Kullman, L., Winterhalter, M. & Bezrukov, S. M. Transport of maltodextrins through maltoporin: a single-channel study. Biophys. J. 82, 803–812 (2002).
Gutsmann, T., Heimburg, T., Keyser, U., Mahendran, K. R. & Winterhalter, M. Protein reconstitution into freestanding planar lipid membranes for electrophysiological characterization. Nat. Protoc. 10, 188–198 (2015).
Mahendran, K. R., Kreir, M., Weingart, H., Fertig, N. & Winterhalter, M. Permeation of antibiotics through Escherichia coli OmpF and OmpC porins: screening for influx on a single-molecule level. J. Biomol. Screen. 15, 302–307 (2010).
Mahendran, K. R. et al. Molecular basis of enrofloxacin translocation through OmpF, an outer membrane channel of Escherichia coli - When binding does not imply translocation. J. Phys. Chem. B 114, 5170–5179 (2010).
Singh, P. R., Ceccarelli, M., Lovelle, M., Winterhalter, M. & Mahendran, K. R. Antibiotic permeation across the OmpF channel: modulation of the affinity site in the presence of magnesium. J. Phys. Chem. B 116, 4433–4438 (2012).
Wang, J., Bafna, J. A., Bhamidimarri, S. P. & Winterhalter, M. Small-molecule permeation across membrane channels: chemical modification to quantify transport across OmpF. Angew. Chem. Int. Ed. 58, 4737–4741 (2019).
Ghai, I. et al. Ampicillin permeation across OmpF, the major outer-membrane channel in Escherichia coli. J. Biol. Chem. 293, 7030–7037 (2018).
Ghai, I. et al. General method to determine the flux of charged molecules through nanopores applied to β-lactamase inhibitors and OmpF. J. Phys. Chem. Lett. 8, 1295–1301 (2017).
Winterhalter, M. & Ceccarelli, M. Physical methods to quantify small antibiotic molecules uptake into Gram-negative bacteria. Eur. J. Pharm. Biopharm. 95, 63–67 (2015).
Scorciapino, M. A. et al. Rationalizing the permeation of polar antibiotics into Gram-negative bacteria. J. Phys. Condens. Matter 29, 113001 (2017).
Tieleman, D. P. & Berendsen, H. J. A molecular dynamics study of the pores formed by Escherichia coli OmpF porin in a fully hydrated palmitoyloleoylphosphatidylcholine bilayer. Biophys. J. 74, 2786–2801 (1998).
Im, W. & Roux, B. Ion permeation and selectivity of OmpF porin: a theoretical study based on molecular dynamics, brownian dynamics, and continuum electrodiffusion theory. J. Mol. Biol. 322, 851–869 (2002).
Aguilella-Arzo, M., García-Celma, J. J., Cervera, J., Alcaraz, A. & Aguilella, V. M. Electrostatic properties and macroscopic electrodiffusion in OmpF porin and mutants. Bioelectrochemistry 70, 320–327 (2007).
Biró, I., Pezeshki, S., Weingart, H., Winterhalter, M. & Kleinekathöfer, U. Comparing the temperature-dependent conductance of the two structurally similar E. coli porins OmpC and OmpF. Biophys. J. 98, 1830–1839 (2010).
Tran, Q.-T., Williams, S., Farid, R., Erdemli, G. & Pearlstein, R. The translocation kinetics of antibiotics through porin OmpC: insights from structure-based solvation mapping using WaterMap. Proteins 81, 291–299 (2013).
Ceccarelli, M., Danelon, C., Laio, A. & Parrinello, M. Microscopic mechanism of antibiotics translocation through a porin. Biophys. J. 87, 58–64 (2004).
Masi, M., Pagès, J.-M. & Winterhalter, M. Outer membrane porins. in Bacterial Cell Walls and Membranes (ed. Kuhn, A.) 79–123 (Springer, 2019).
Acosta-Gutierrez, S., Scorciapino, M. A., Bodrenko, I. & Ceccarelli, M. Filtering with electric field: the case of E. coli porins. J. Phys. Chem. Lett. 6, 1807–1812 (2015).
Bajaj, H. et al. Molecular basis of filtering carbapenems by porins from β-lactam-resistant clinical strains of Escherichia coli. J. Biol. Chem. 291, 2837–2847 (2016).
Acosta-Gutierrez, S., Bodrenko, I., Scorciapino, M. A. & Ceccarelli, M. Macroscopic electric field inside water-filled biological nanopores. Phys. Chem. Chem. Phys. 18, 8855–8864 (2016).
Kojima, S. & Nikaido, H. High salt concentrations increase permeability through OmpC channels of Escherichia coli. J. Biol. Chem. 289, 26464–26473 (2014).
D’Agostino, T., Salis, S. & Ceccarelli, M. A kinetic model for molecular diffusion through pores. Biochim. Biophys. Acta 1858, 1772–1777 (2016).
Bodrenko, I., Bajaj, H., Ruggerone, P., Winterhalter, M. & Ceccarelli, M. Analysis of fast channel blockage: revealing substrate binding in the microsecond range. Analyst 140, 4820–4827 (2015).
Bodrenko, I. V., Wang, J., Salis, S., Winterhalter, M. & Ceccarelli, M. Sensing single molecule penetration into nanopores: pushing the time resolution to the diffusion limit. ACS Sens. 2, 1184–1190 (2017).
Bajaj, H. et al. Bacterial outer membrane porins as electrostatic nanosieves: exploring transport rules of small polar molecules. ACS Nano 11, 5465–5473 (2017).
Payne, D. J., Gwynn, M. N., Holmes, D. J. & Pompliano, D. L. Drugs for bad bugs: confronting the challenges of antibacterial discovery. Nat. Rev. Drug Discov. 6, 29–40 (2007).
O’Shea, R. & Moser, H. E. Physicochemical properties of antibacterial compounds: Implications for drug discovery. J. Med. Chem. 51, 2871–2878 (2008).
Brown, D. G., May-Dracka, T. L., Gagnon, M. M. & Tommasi, R. Trends and exceptions of physical properties on antibacterial activity for gram-positive and gram-negative pathogens. J. Med. Chem. 57, 10144–10161 (2014).
Page, M. G. P. Beta-lactam antibiotics. in Antibiotic Discovery and Development (eds Dougherty, T. J. & Pucci, M.) 79–117 (Springer, 2011).
Bodrenko, I. V., Salis, S., Acosta-Gutierrez, S. & Ceccarelli, M. Diffusion of large particles through small pores: from entropic to enthalpic transport. J. Chem. Phys. 150, 211102–211106 (2019).
Grabowicz, M. & Silhavy, T. J. Envelope stress responses: an interconnected safety net. Trends Biochem. Sci. 42, 232–242 (2017).
Chen, H. D. & Groisman, E. A. The biology of the PmrA/PmrB two-component system: the major regulator of lipopolysaccharide modifications. Annu. Rev. Microbiol. 67, 83–112 (2013).
Guest, R. L. & Raivio, T. L. Role of the Gram-negative envelope stress response in the presence of antimicrobial agents. Trends Microbiol. 24, 377–390 (2016).
Zschiedrich, C. P., Keidel, V. & Szurmant, H. Molecular mechanisms of two-component signal transduction. J. Mol. Biol. 428, 3752–3775 (2016).
Laloux, G. & Collet, J.-F. Major Tom to ground control: how lipoproteins communicate extracytoplasmic stress to the decision center of the cell. J. Bacteriol. 199, e00216–e00217 (2017).
Tiwari, S. et al. Two-component signal transduction systems of pathogenic bacteria as targets for antimicrobial therapy: an overview. Front. Microbiol. 8, 1878 (2017).
Fröhlich, K. S. & Gottesman, S. Small regulatory RNAs in the enterobacterial response to envelope damage and oxidative stress. Microbiol. Spectr. 6, RWR-0022-2018 (2018).
Barquist, L. & Vogel, J. Accelerating discovery and functional analysis of small RNAs with new technologies. Annu. Rev. Genet. 49, 367–394 (2015).
Vogel, J. & Wagner, E. G. H. Target identification of small noncoding RNAs in bacteria. Curr. Opin. Microbiol. 10, 262–270 (2007).
Guillier, M., Gottesman, S. & Storz, G. Modulating the outer membrane with small RNAs. Genes Dev. 20, 2338–2348 (2006).
Vogel, J. & Papenfort, K. Small non-coding RNAs and the bacterial outer membrane. Curr. Opin. Microbiol. 9, 605–611 (2006).
Valentin-Hansen, P., Johansen, J. & Rasmussen, A. A. Small RNAs controlling outer membrane porins. Curr. Opin. Microbiol. 10, 152–155 (2007).
Delihas, N. & Forst, S. MicF: an antisense RNA gene involved in response of Escherichia coli to global stress factors. J. Mol. Biol. 313, 1–12 (2001).
Andersen, J. & Delihas, N. micF RNA binds to the 5’ end of ompF mRNA and to a protein from Escherichia coli. Biochemistry 29, 9249–9256 (1990).
Delihas, N. Discovery and characterization of the first non-coding RNA that regulates gene expression, micF RNA: a historical perspective. World J. Biol. Chem. 6, 272–280 (2015).
Inouye, M. The first demonstration of RNA interference to inhibit mRNA function. Gene 592, 332–333 (2016).
Chen, S., Zhang, A., Blyn, L. B. & Storz, G. MicC, a second small-RNA regulator of Omp protein expression in Escherichia coli. J. Bacteriol. 186, 6689–6697 (2004).
Dam, S., Pagès, J.-M. & Masi, M. Dual regulation of the small RNA MicC and the quiescent porin OmpN in response to antibiotic stress in Escherichia coli. Antibiotics 6, 33 (2017).
Ramani, N., Hedeshian, M. & Freundlich, M. micF antisense RNA has a major role in osmoregulation of OmpF in Escherichia coli. J. Bacteriol. 176, 5005–5010 (1994).
Chou, J. H., Greenberg, J. T. & Demple, B. Posttranscriptional repression of Escherichia coli OmpF protein in response to redox stress: positive control of the micF antisense RNA by the soxRS locus. J. Bacteriol. 175, 1026–1031 (1993).
Davin-Regli, A. et al. Membrane permeability and regulation of drug “influx and efflux” in enterobacterial pathogens. Curr. Drug Targets 9, 750–759 (2008).
Blair, J. M. A., Webber, M. A., Baylay, A. J., Ogbolu, D. O. & Piddock, L. J. V. Molecular mechanisms of antibiotic resistance. Nat. Rev. Microbiol. 13, 42–51 (2015).
Du, D. et al. Multidrug efflux pumps: structure, function and regulation. Nat. Rev. Microbiol. 16, 523 (2018).
Dupont, H. et al. Structural alteration of OmpR as a source of ertapenem resistance in a CTX-M-15-producing Escherichia coli O25b:H4 sequence type 131 clinical isolate. Antimicrob. Agents Chemother. 61, e00014–e00017 (2017).
Dupont, H. et al. Molecular characterization of carbapenem-nonsusceptible enterobacterial isolates collected during a prospective interregional survey in France and susceptibility to the novel ceftazidime-avibactam and aztreonam-avibactam combinations. Antimicrob. Agents Chemother. 60, 215–221 (2016).
Tran, Q.-T. et al. Implication of porins in β-lactam resistance of Providencia stuartii. J. Biol. Chem. 285, 32273–32281 (2010).
Tran, Q.-T. et al. Porin flexibility in Providencia stuartii: cell-surface-exposed loops L5 and L7 are markers of Providencia porin OmpPst1. Res. Microbiol. 168, 685–699 (2017).
Oteo, J. et al. Emergence of imipenem resistance in clinical Escherichia coli during therapy. Int. J. Antimicrob. Agents 32, 534–537 (2008).
Philippe, N. et al. In vivo evolution of bacterial resistance in two cases of Enterobacter aerogenes infections during treatment with imipenem. PLOS ONE 10, e0138828 (2015).
Lázaro-Perona, F. et al. Genomic path to pandrug resistance in a clinical isolate of Klebsiella pneumoniae. Int. J. Antimicrob. Agents 52, 713–718 (2018).
Yang, F.-C., Yan, J.-J., Hung, K.-H. & Wu, J.-J. Characterization of ertapenem-resistant Enterobacter cloacae in a Taiwanese University Hospital. J. Clin. Microbiol. 50, 223–226 (2012).
Fernández, J., Guerra, B. & Rodicio, M. R. Resistance to carbapenems in non-typhoidal Salmonella enterica Serovars from humans, animals and food. Vet. Sci. 5, E40 (2018).
Doumith, M., Ellington, M. J., Livermore, D. M. & Woodford, N. Molecular mechanisms disrupting porin expression in ertapenem-resistant Klebsiella and Enterobacter spp. clinical isolates from the UK. J. Antimicrob. Chemother. 63, 659–667 (2009).
Wozniak, A. et al. Porin alterations present in non-carbapenemase-producing Enterobacteriaceae with high and intermediate levels of carbapenem resistance in Chile. J. Med. Microbiol. 61, 1270–1279 (2012).
Cerqueira, G. C. et al. Multi-institute analysis of carbapenem resistance reveals remarkable diversity, unexplained mechanisms, and limited clonal outbreaks. Proc. Natl Acad. Sci. USA 114, 1135–1140 (2017).
Novais, Â. et al. Spread of an OmpK36-modified ST15 Klebsiella pneumoniae variant during an outbreak involving multiple carbapenem-resistant Enterobacteriaceae species and clones. Eur. J. Clin. Microbiol. Infect. Dis. 31, 3057–3063 (2012).
Shin, S. Y. et al. Resistance to carbapenems in sequence type 11 Klebsiella pneumoniae is related to DHA-1 and loss of OmpK35 and/or OmpK36. J. Med. Microbiol. 61, 239–245 (2012).
Martínez-Martínez, L. Extended-spectrum beta-lactamases and the permeability barrier. Clin. Microbiol. Infect. 14, 82–89 (2008).
Thiolas, A., Bollet, C., Scola, B. L., Raoult, D. & Pagès, J.-M. Successive emergence of Enterobacter aerogenes strains resistant to imipenem and colistin in a patient. Antimicrob. Agents Chemother. 49, 1354–1358 (2005).
Lavigne, J.-P. et al. An adaptive response of Enterobacter aerogenes to imipenem: regulation of porin balance in clinical isolates. Int. J. Antimicrob. Agents 41, 130–136 (2013).
Lavigne, J.-P. et al. Membrane permeability, a pivotal function involved in antibiotic resistance and virulence in Enterobacter aerogenes clinical isolates. Clin. Microbiol. Infect. 18, 539–545 (2012).
Hamzaoui, Z. et al. An outbreak of NDM-1-producing Klebsiella pneumoniae, associated with OmpK35 and OmpK36 porin loss in Tunisia. Microb. Drug Resist. 24, 1137–1147 (2018).
Gröbner, S. et al. Emergence of carbapenem-non-susceptible extended-spectrum β-lactamase-producing Klebsiella pneumoniae isolates at the university hospital of Tübingen, Germany. J. Med. Microbiol. 58, 912–922 (2009).
Findlay, J., Hamouda, A., Dancer, S. J. & Amyes, S. G. B. Rapid acquisition of decreased carbapenem susceptibility in a strain of Klebsiella pneumoniae arising during meropenem therapy. Clin. Microbiol. Infect. 18, 140–146 (2012).
Nelson, K. et al. Resistance to ceftazidime-avibactam is due to transposition of KPC in a porin-deficient strain of Klebsiella pneumoniae with increased efflux activity. Antimicrob. Agents Chemother. 61, e00989–17 (2017).
Shen, Z. et al. High ceftazidime hydrolysis activity and porin OmpK35 deficiency contribute to the decreased susceptibility to ceftazidime/avibactam in KPC-producing Klebsiella pneumoniae. J. Antimicrob. Chemother. 72, 1930–1936 (2017).
Pagès, J.-M., Peslier, S., Keating, T. A., Lavigne, J.-P. & Nichols, W. W. Role of the outer membrane and porins in susceptibility of β-lactamase-producing Enterobacteriaceae to ceftazidime-avibactam. Antimicrob. Agents Chemother. 60, 1349–1359 (2016).
Balabanian, G., Rose, M., Manning, N., Landman, D. & Quale, J. Effect of porins and bla KPC expression on activity of imipenem with relebactam in Klebsiella pneumoniae: Can antibiotic combinations overcome resistance? Microb. Drug Resist. 24, 877–881 (2018).
Sun, D., Rubio-Aparicio, D., Nelson, K., Dudley, M. N. & Lomovskaya, O. Meropenem-vaborbactam resistance selection, resistance prevention, and molecular mechanisms in mutants of KPC-producing Klebsiella pneumoniae. Antimicrob. Agents Chemother. 61, e01694-17 (2017).
Lunha, K. et al. High-level carbapenem-resistant OXA-48-producing Klebsiella pneumoniae with a novel OmpK36 variant and low-level, carbapenem-resistant, non-porin-deficient, OXA-181-producing Escherichia coli from Thailand. Diagn. Microbiol. Infect. Dis. 85, 221–226 (2016).
Dé, E. et al. A new mechanism of antibiotic resistance in Enterobacteriaceae induced by a structural modification of the major porin. Mol. Microbiol. 41, 189–198 (2001).
Thiolas, A., Bornet, C., Davin-Régli, A., Pagès, J.-M. & Bollet, C. Resistance to imipenem, cefepime, and cefpirome associated with mutation in Omp36 osmoporin of Enterobacter aerogenes. Biochem. Biophys. Res. Commun. 317, 851–856 (2004).
García-Fernández, A. et al. An ertapenem-resistant extended-spectrum-β-lactamase-producing Klebsiella pneumoniae clone carries a novel OmpK36 porin variant. Antimicrob. Agents Chemother. 54, 4178–4184 (2010).
Clancy, C. J. et al. Mutations of the ompK36 porin gene and promoter impact responses of sequence type 258, KPC-2-producing Klebsiella pneumoniae strains to doripenem and doripenem-colistin. Antimicrob. Agents Chemother. 57, 5258–5265 (2013).
Papagiannitsis, C. C. et al. OmpK35 and OmpK36 porin variants associated with specific sequence types of Klebsiella pneumoniae. J. Chemother. 25, 250–254 (2013).
Hall, J. M., Corea, E., Sanjeewani, H. D. A. & Inglis, T. J. J. Molecular mechanisms of β-lactam resistance in carbapenemase-producing Klebsiella pneumoniae from Sri Lanka. J. Med. Microbiol. 63, 1087–1092 (2014).
Partridge, S. R. et al. Emergence of bla KPC carbapenemase genes in Australia. Int. J. Antimicrob. Agents 45, 130–136 (2015).
Knopp, M. & Andersson, D. I. Amelioration of the fitness costs of antibiotic resistance due to reduced outer membrane permeability by upregulation of alternative porins. Mol. Biol. Evol. 32, 3252–3263 (2015).
García-Sureda, L., Juan, C., Doménech-Sánchez, A. & Albertí, S. Role of Klebsiella pneumoniae LamB porin in antimicrobial resistance. Antimicrob. Agents Chemother. 55, 1803–1805 (2011).
Bialek-Davenet, S. et al. In-vivo loss of carbapenem resistance by extensively drug-resistant Klebsiella pneumoniae during treatment via porin expression modification. Sci. Rep. 7, 6722 (2017).
Kaczmarek, F. M., Dib-Hajj, F., Shang, W. & Gootz, T. D. High-level carbapenem resistance in a Klebsiella pneumoniae clinical isolate is due to the combination of bla ACT-1 β-lactamase production, porin OmpK35/36 insertional inactivation, and down-regulation of the phosphate transport porin PhoE. Antimicrob. Agents Chemother. 50, 3396–3406 (2006).
García-Sureda, L. et al. OmpK26, a novel porin associated with carbapenem resistance in Klebsiella pneumoniae. Antimicrob. Agents Chemother. 55, 4742–4747 (2011).
Doménech-Sánchez, A., Hernández-Allés, S., Martínez-Martínez, L., Benedí, V. J. & Albertí, S. Identification and characterization of a new porin gene of Klebsiella pneumoniae: Its role in β-lactam antibiotic resistance. J. Bacteriol. 181, 2726–2732 (1999).
Castanheira, M., Mendes, R. E. & Sader, H. S. Low frequency of ceftazidime-avibactam resistance among Enterobacteriaceae isolates carrying bla KPC collected in U.S. hospitals from 2012 to 2015. Antimicrob. Agents Chemother. 61, e02369-16 (2017).
Noinaj, N., Gumbart, J. C. & Buchanan, S. K. The β-barrel assembly machinery in motion. Nat. Rev. Microbiol. 15, 197–204 (2017).
Schiffrin, B., Brockwell, D. J. & Radford, S. E. Outer membrane protein folding from an energy landscape perspective. BMC Biol. 15, 123 (2017).
Robinson, J. A. Folded synthetic peptides and other molecules targeting outer membrane protein complexes in gram-negative bacteria. Front. Chem. 7, 45 (2019).
Hao, M. et al. Porin deficiency in carbapenem-resistant Enterobacter aerogenes strains. Microb. Drug Resist. 24, 1277–1283 (2018).
Jiménez-Castellanos, J.-C. et al. Envelope proteome changes driven by RamA overproduction in Klebsiella pneumoniae that enhance acquired β-lactam resistance. J. Antimicrob. Chemother. 73, 88–94 (2018).
Chetri, S. et al. Transcriptional response of OmpC and OmpF in Escherichia coli against differential gradient of carbapenem stress. BMC Res. Notes 12, 138 (2019).
Bhamidimarri, S. P., Prajapati, J. D., van den Berg, B., Winterhalter, M. & Kleinekathöfer, U. Role of electroosmosis in the permeation of neutral molecules: CymA and cyclodextrin as an example. Biophys. J. 110, 600–611 (2016).
Olivares, J. et al. The intrinsic resistome of bacterial pathogens. Front. Microbiol. 4, 103 (2013).
Li, X.-Z., Plésiat, P. & Nikaido, H. The challenge of efflux-mediated antibiotic resistance in gram-negative bacteria. Clin. Microbiol. Rev. 28, 337–418 (2015).
Xia, J., Gao, J. & Tang, W. Nosocomial infection and its molecular mechanisms of antibiotic resistance. Biosci. Trends 10, 14–21 (2016).
Nikaido, H. & Pagès, J.-M. Broad specificity efflux pumps and their role in multidrug resistance of gram negative bacteria. FEMS Microbiol. Rev. 36, 340–363 (2012).
Davin-Regli, A., Lavigne, J.-P. & Pagès, J.-M. Enterobacter spp.: update on taxonomy, clinical aspects, and emerging antimicrobial resistance. Clin. Microbiol. Rev. 32, e00002-e00019 (2019).
Pagès, J.-M. et al. Efflux Pump, the masked side of ß-Lactam resistance in Klebsiella pneumoniae clinical isolates. PLOS ONE 4, e4817 (2009).
Wan Nur Ismah, W. A. K., Takebayashi, Y., Findlay, J., Heesom, K. J. & Avison, M. B. Impact of OqxR loss of function on the envelope proteome of Klebsiella pneumoniae and susceptibility to antimicrobials. J. Antimicrob. Chemother. 73, 2990–2996 (2018).
Nikaido, H. Role of permeability barriers in resistance to β-lactam antibiotics. Pharmacol. Ther. 27, 197–231 (1985).
Talbot, G. H. β-Lactam antimicrobials: what have you done for me lately? Ann. N. Y. Acad. Sci. 1277, 76–83 (2013).
Westfall, D. A. et al. Bifurcation kinetics of drug uptake by Gram-negative bacteria. PLOS ONE 12, e0184671 (2017).
Nicolas-Chanoine, M.-H., Mayer, N., Guyot, K., Dumont, E. & Pagès, J.-M. Interplay between membrane permeability and enzymatic barrier leads to antibiotic-dependent resistance in Klebsiella pneumoniae. Front. Microbiol. 9, 1422 (2018).
Castanheira, M. et al. Analyses of a ceftazidime-avibactam-resistant Citrobacter freundii isolate carrying bla KPC-2 reveals a heterogenous population and reversible genotype. mSphere 3, e00408-18 (2018).
Fajardo-Lubián, A., Ben Zakour, N. L., Agyekum, A., Qi, Q. & Iredell, J. R. Host adaptation and convergent evolution increases antibiotic resistance without loss of virulence in a major human pathogen. PLOS Pathog. 15, e1007218 (2019).
Coines, J., Acosta-Gutierrez, S., Bodrenko, I., Rovira, C. & Ceccarelli, M. Glucose transport via the pseudomonads porin OprB. Implications for the design of Trojan-horse antinfectives. Phys. Chem. Chem. Phys. 21, 8457–8463 (2019).
Acknowledgements
The authors gratefully acknowledge R. A. Stavenger for stimulating and helpful discussions. The research leading to this article was conducted as part of the TRANSLOCATION consortium, and it has received support from the Innovative Medicines Initiatives Joint Undertaking under Grant Agreement no. 115525, resources that are composed of financial contributions from the European Union’s seventh framework programme (FP7/2007–2013) and the European Federation of Pharmaceutical Industries and Associations companies in kind contribution. J.M.P. was also partially supported by Aix-Marseille Université, Service de Santé des Armées and INSERM. M.C. was partially supported by the Italian MIUR, PRIN Project 2015795S5W_005. L.M. was also supported by the ND4BB ENABLE Consortium under grant agreement no. 115583. The authors apologize to those researchers whose articles are not cited owing to space limitations.
Reviewer information
Nature Reviews Microbiology thanks H. Zgurskaya, S. Bezrukov and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
All authors researched data for article. J.-M.P., J.V., I.V.B., M.M., L.M., J.H.N., A.D.-R., M.C., B.v.d.B. and M.W. contributed substantially to the discussion of the content. J.-M.P., J.V., I.V.B., M.M., L.M., S.A.-G., J.H.N., M.C., B.v.d.B. and M.W. wrote the article. and J.-M.P., J.V., J.H.N., M.C., B.v.d.B. and M.W. reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related link
TRANSLOCATION consortium: https://www.imi.europa.eu/projects-results/project-factsheets/translocation
Supplementary information
Glossary
- β-barrels
-
β-barrels comprise an even number of β-strands, ranging from 8 to 24, that are arranged in an antiparallel fashion.
- β-Lactamases
-
Bacterial enzymes located in the periplasm of Gram-negative bacteria that cleave β-lactam antibiotics such as penicillins, cephalosporins and carbapenems.
- Counterion
-
Ion accompanying a charged amino acid to maintain charge neutrality. Whereas the amino acid is fixed, the counterion is solubilized in the aqueous phase and moves with the electric field.
- Graphics processing units
-
Hardware components of a computer able to increase computational speed by performing parallel calculations. They are typically used for gaming, but their use has been extended to use by scientific software that runs much faster (a factor of 10–100) than on a normal central processing unit.
- Dipole moment
-
Electric dipole moment applied to compounds or molecules. It provides information on the distribution of charges in the scaffold. A zwitterionic molecule possesses a large dipole when the two charged groups are far apart.
Rights and permissions
About this article
Cite this article
Vergalli, J., Bodrenko, I.V., Masi, M. et al. Porins and small-molecule translocation across the outer membrane of Gram-negative bacteria. Nat Rev Microbiol 18, 164–176 (2020). https://doi.org/10.1038/s41579-019-0294-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41579-019-0294-2
This article is cited by
-
Plasma Sterilization for Bacterial Inactivation: Studies on Probable Mechanisms and Biochemical Actions
Plasma Chemistry and Plasma Processing (2024)
-
Size and charge effects of metal nanoclusters on antibacterial mechanisms
Journal of Nanobiotechnology (2023)
-
Strong chemotaxis by marine bacteria towards polysaccharides is enhanced by the abundant organosulfur compound DMSP
Nature Communications (2023)
-
Collateral sensitivity profiling in drug-resistant Escherichia coli identifies natural products suppressing cephalosporin resistance
Nature Communications (2023)
-
Protein nanopore reveals the renin–angiotensin system crosstalk with single-amino-acid resolution
Nature Chemistry (2023)