Abstract
Purpose
To summarise data with radium-223 dichloride (223RaCl2), a mechanism-mediated targeted alpha therapy (TAT), in metastatic castration-resistant prostate cancer (mCRPC) and to chart the development of TAT in mCRPC and in other tumour types.
Methods
Literature for this systematic review was identified using a PubMed search: (“targeted alpha therapy” or “targeted alpha particle therapy”) or (213-bismuth or bismuth-213 or 213Bi) or (225-actinium or actinium-225 or 225Ac) or (211-astatine or astatine-211 or 211At) or (212-lead or lead-212 or 212Pb) or (227-thorium or thorium-227 or 227Th) or (223-radium or radium-223 or 223Ra or alpharadin) and (malignancy or cancer). Results were limited to English-language publications in humans, with the article type “clinical trial”.
Results
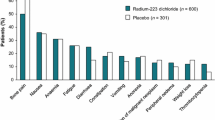
Forty-one publications were included (30 from the literature search and 11 from manual searches/reviews). In clinical trials in mCRPC, 223RaCl2 monotherapy is well tolerated, with significantly longer overall survival than placebo and improved quality of life. Clinical trial data have been reinforced by findings from real-world studies. 223RaCl2 has also shown promise in other tumour types with bone metastases, including advanced breast cancer and advanced renal cell carcinoma (in combination with anti-vascular endothelial growth factor). Several astatine-211- and bismuth-213-labelled molecules have demonstrated anti-tumour activity and acceptable toxicity in other tumour types.
Conclusions
223RaCl2 has demonstrated “proof of concept” for use of TAT in cancer in clinical practice. The efficacy and safety of 223RaCl2 monotherapy have been demonstrated in mCRPC, and 223RaCl2 combination therapies are under investigation in various tumours. TAT has broad applicability across tumour types.


Similar content being viewed by others
Abbreviations
- 225Ac:
-
actinium-225
- 211At:
-
astatine-211
- 213Bi:
-
bismuth-213
- 212Pb:
-
lead-212
- 223RaCl2 :
-
radium-223 dichloride
- 227Th:
-
thorium-227
- AE:
-
adverse event
- AJCC/UICC:
-
American Joint Committee on Cancer /Union for International Cancer Control
- ALP:
-
alkaline phosphatase
- ALL:
-
acute lymphocytic leukaemia
- ALSYMPCA:
-
Alpharadin in Symptomatic Prostate Cancer
- ALT:
-
alanine transaminase
- AML:
-
acute myeloid leukaemia
- AST:
-
aspartate aminotransferase
- CI:
-
confidence interval
- CLAG-M:
-
cladribine, cytarabine, granulocyte-colony stimulating factor, mitoxantrone
- DNA:
-
deoxyribonucleic acid
- EAP:
-
Early Access Programme
- ECOG:
-
Eastern Cooperative Oncology Group
- EGFR:
-
epidermal growth factor receptor
- EMA:
-
European Medicines Agency
- EOBD:
-
extent of bone disease category
- EQ-5D:
-
EuroQoL 5D
- FACT-P:
-
Functional Assessment of Cancer Therapy-Prostate
- FDA:
-
Food and Drug Administration
- HAMA:
-
human-antimouse-antibody
- HER2:
-
human epidermal growth factor 2
- HR:
-
hazard ratio
- LDH:
-
lactate dehydrogenase
- LNRH:
-
luteinising hormone-releasing hormone
- mCRPC:
-
metastatic castration-resistant prostate cancer
- MHLW:
-
Ministry of Health, Labour and Welfare
- MTD:
-
maximum tolerated dose
- OS:
-
overall survival
- PSA:
-
prostate-specific antigen
- PSMA:
-
prostate-specific membrane antigen
- QOL:
-
quality of life
- RANKL:
-
receptor activator of nuclear factor-kappaB ligand
- REASSURE:
-
Radium-223 Alpha Emitter Agent in Non-intervention Safety Study in mCRPC popUlation for Long-teRm Evaluation
- SSRE:
-
symptomatic skeleton-related events
- TAT:
-
targeted alpha therapy
- TCMC:
-
S-2-(4-isothiocyanatobenzyl)-1,4,7,10-tetraaza-1,4,7,10-tetra(2-carbamoylmethyl)cyclododecane
- TEAE:
-
treatment-emergent adverse event
- ULN:
-
upper limit of normal
- WHO:
-
World Health Organization
References
Sofou S. Radionuclide carriers for targeting of cancer. Int J Nanomedicine. 2008;3:181–99.
Marcu L, Bezak E, Allen BJ. Global comparison of targeted alpha vs targeted beta therapy for cancer: in vitro, in vivo and clinical trials. Crit Rev Oncol Hematol. 2018;123:7–20.
Sartor O, Sharma D. Radium and other alpha emitters in prostate cancer. Transl Androl Urol. 2018;7:436–44.
Bayer. Xofigo Summary of Product Characteristics. 2018. Available from: https://www.medicines.org.uk/emc/product/5204/smpc. Accessed 13 Dec 2018.
Bayer. Xofigo Prescribing Information. 2018. Available from: http://labeling.bayerhealthcare.com/html/products/pi/Xofigo_PI.pdf. Accessed 13 Dec 2018.
Pharmaceuticals and Medical Devices Agency Japan. Annual Report FY 2015. Available from: https://ss.pmda.go.jp/en_all/muv_ajax.x?u=http%3A%2F%2Fwww.pmda.go.jp%2Ffiles%2F000214925.pdf%23page%3D195&p=195&t=&q=xofigo&s=qV0C6RpV7dquA8XpExigITDmeas4Getv3NRnOl7tdNESUBPUMP1Od-2SPxc1wnSjSIZf5FhtuhkxQjzWifiv4-7EB0KxSmNdQOYk8A4MmU-SaochybEWcUKDVQvXVmnB. Accessed 20 Feb 2019.
Nilsson S, Larsen RH, Fosså SD, Balteskard L, Borch KW, Westlin J-E, et al. First clinical experience with alpha-emitting radium-223 in the treatment of skeletal metastases. Clin Cancer Res. 2005;11:4451–9.
Chittenden SJ, Hindorf C, Parker CC, Lewington VJ, Pratt BE, Johnson B, et al. A phase 1, open-label study of the biodistribution, pharmacokinetics, and dosimetry of 223Ra-dichloride in patients with hormone-refractory prostate cancer and skeletal metastases. J Nucl Med. 2015;56:1304–9.
Nilsson S, Franzén L, Parker C, Tyrrell C, Blom R, Tennvall J, et al. Bone-targeted radium-223 in symptomatic, hormone-refractory prostate cancer: a randomised, multicentre, placebo-controlled phase II study. Lancet Oncol. 2007;8:587–94.
Nilsson S, Strang P, Aksnes AK, Franzèn L, Olivier P, Pecking A, et al. A randomized, dose-response, multicenter phase II study of radium-223 chloride for the palliation of painful bone metastases in patients with castration-resistant prostate cancer. Eur J Cancer. 2012;48:678–86.
Parker C, Nilsson S, Heinrich D, Helle SI, O’Sullivan JM, Fosså SD, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369:213–23.
Parker C, Coleman R, Sartor O, Vogelzang N, Bottomley D, Heinrich D, et al. Three-year safety of radium-223 dichloride in patients with castration-resistant prostate cancer and symptomatic bone metastases from phase 3 randomized alpharadin in symptomatic prostate cancer trial. Eur Urol. 2018;73:427–35.
Sartor O, Coleman R, Nilsson S, Heinrich D, Helle SI, O’Sullivan JM, et al. Effect of radium-223 dichloride on symptomatic skeletal events in patients with castration-resistant prostate cancer and bone metastases: results from a phase 3, double-blind, randomised trial. Lancet Oncol. 2014;15:738–46.
Sartor O, Coleman RE, Nilsson S, Heinrich D, Helle SI, O’Sullivan JM, et al. An exploratory analysis of alkaline phosphatase, lactate dehydrogenase, and prostate-specific antigen dynamics in the phase 3 ALSYMPCA trial with radium-223. Ann Oncol. 2017;28:1090–7.
Parker C, Zhan L, Cislo P, Reuning-Scherer J, Vogelzang NJ, Nilsson S, et al. Effect of radium-223 dichloride (Ra-223) on hospitalisation: an analysis from the phase 3 randomised Alpharadin in Symptomatic Prostate Cancer Patients (ALSYMPCA) trial. Eur J Cancer. 2017;71:1–6.
Parker C, Finkelstein SE, Michalski JM, O’Sullivan JM, Bruland Ø, Vogelzang NJ, et al. Efficacy and safety of radium-223 dichloride in symptomatic castration-resistant prostate cancer patients with or without baseline opioid use from the phase 3 ALSYMPCA trial. Eur Urol. 2016;70:875–83.
Hoskin P, Sartor O, O’Sullivan JM, Johannessen DC, Helle SI, Logue J, et al. Efficacy and safety of radium-223 dichloride in patients with castration-resistant prostate cancer and symptomatic bone metastases, with or without previous docetaxel use: a prespecified subgroup analysis from the randomised, double-blind, phase 3 ALSYMPC. Lancet Oncol. 2014;15:1397–406.
Sartor O, Hoskin P, Coleman RE, Nilsson S, Vogelzang NJ, Petrenciuc O, et al. Chemotherapy following radium-223 dichloride treatment in ALSYMPCA. Prostate. 2016;76:905–16.
Vogelzang NJ, Coleman RE, Michalski JM, Nilsson S, O’Sullivan JM, Parker C, et al. Hematologic safety of radium-223 dichloride: baseline prognostic factors associated with myelosuppression in the ALSYMPCA Trial. Clin Genitourin Cancer. 2017;15:42–52.e8.
Nilsson S, Cislo P, Sartor O, Vogelzang NJ, Coleman RE, O’Sullivan JM, et al. Patient-reported quality-of-life analysis of radium-223 dichloride from the phase III ALSYMPCA study. Ann Oncol Off J Eur Soc Med Oncol. 2016;27:868–74.
Uemura H, Uemura H, Matsubara N, Kinuya S, Hosono M, Yajima Y, et al. Safety and efficacy of radium-223 dichloride in Japanese patients with castration-resistant prostate cancer and bone metastases. Int J Clin Oncol. 2017;22:954–63.
Matsubara N, Nagamori S, Wakumoto Y, Uemura H, Kimura G, Yokomizo A, et al. Phase II study of radium-223 dichloride in Japanese patients with symptomatic castration-resistant prostate cancer. Int J Clin Oncol. 2018;23:173–80.
Saad F, Carles J, Gillessen S, Heidenreich A, Heinrich D, Gratt J, et al. Radium-223 and concomitant therapies in patients with metastatic castration-resistant prostate cancer: an international, early access, open-label, single-arm phase 3b trial. Lancet Oncol. 2016;17:1306–16.
Carles J, Méndez MJ, Pinto Á, Sáez MI, Arranz JA, Maroto P, et al. Radium-223 international early access program: results from the Spanish subset. Future Oncol. 2018;14:41–50.
Dizdarevic S, Meidahl Petersen P, Essler M, Versari A, Bourre J-C, la Fougere C, et al. Interim analysis of the REASSURE (Radium-223 alpha Emitter Agent in non-intervention Safety Study in mCRPC popUlation for long-teRm Evaluation) study: patient characteristics and safety according to prior use of chemotherapy in routine clinical practice. 2019;46:1102–10.
Miederer M, Thomas C, Beck J, Hampel C, Krieger C, Baqué PE, et al. Haematopoietic toxicity of radium-223 in patients with high skeletal tumour burden. Nuklearmedizin. 2015;54:197–203.
Sartor O, Heinrich D, Mariados N, Méndez Vidal MJ, Keizman D, Thellenberg Karlsson C, et al. Re-treatment with radium-223: first experience from an international, open-label, phase I/II study in patients with castration-resistant prostate cancer and bone metastases. Ann Oncol Off J Eur Soc Med Oncol. 2017;28:2464–71.
Dizdarevic S, Jessop M, Begley P, Main S, Robinson A. 223Ra-dichloride in castration-resistant metastatic prostate cancer: improving outcomes and identifying predictors of survival in clinical practice. Eur J Nucl Med Mol Imaging. 2018;45:2264–73.
Dan TD, Eldredge-Hindy HB, Hoffman-Censits J, Lin J, Kelly WK, Gomella LG, et al. Hematologic toxicity of concurrent administration of radium-223 and next-generation antiandrogen therapies. Am J Clin Oncol. 2017;40:342–7.
European Medicines Agency. Prostate cancer medicine Xofigo must not be used with Zytiga and prednisone/prednisolone. 2018. Available from: https://www.ema.europa.eu/en/news/prostate-cancer-medicine-xofigo-must-not-be-used-zytiga-prednisoneprednisolone. Accessed 18 Dec 2018.
Shore ND, Tutrone RF, Mariados NF, Nordquist LT, Mehlhaff BA, Steere KJ, et al. eRADicAte: a prospective evaluation combining radium-223 dichloride and abiraterone acetate plus prednisone in patients with castration-resistant prostate cancer. Clin Genitourin Cancer. 2018;16:149–54.
Kratochwil C, Bruchertseifer F, Rathke H, Hohenfellner M, Giesel FL, Haberkorn U, et al. Targeted α-therapy of metastatic castration-resistant prostate cancer with 225Ac-PSMA-617: swimmer-plot analysis suggests efficacy regarding duration of tumor control. J Nucl Med. 2018;59:795–802.
Sathekge M, Bruchertseifer F, Knoesen O, Reyneke F, Lawal I, Lengana T, et al. 225Ac-PSMA-617 in chemotherapy-naive patients with advanced prostate cancer: a pilot study. Eur J Nucl Med Mol Imaging. 2019;46:129–38.
Coleman R, Aksnes A-K, Naume B, Garcia C, Jerusalem G, Piccart M, et al. A phase IIa, nonrandomized study of radium-223 dichloride in advanced breast cancer patients with bone-dominant disease. Breast Cancer Res Treat. 2014;145:411–8.
McKay R, Bosse D, Gray K, Michaelson M, Krajewski K, Jacene H, et al. Radium-223 dichloride in combination with vascular endothelial growth factor-targeting therapy in advanced renal cell carcinoma with bone metastases. Clin Cancer Res. 2018. https://doi.org/10.1158/1078-0432.CCR-17-3577.
Zalutsky MR, Reardon DA, Akabani G, Coleman RE, Friedman AH, Friedman HS, et al. Clinical experience with -particle emitting 211At: treatment of recurrent brain tumor patients with 211At-labeled chimeric antitenascin monoclonal antibody 81C6. J Nucl Med. 2007;49:30–8.
Andersson H, Cederkrantz E, Bäck T, Divgi C, Elgqvist J, Himmelman J, et al. Intraperitoneal alpha-particle radioimmunotherapy of ovarian cancer patients: pharmacokinetics and dosimetry of (211)At-MX35 F(ab’)2--a phase I study. J Nucl Med. 2009;50:1153–60.
Allen BJ, Raja C, Rizvi S, Li Y, Tsui W, Graham P, et al. Intralesional targeted alpha therapy for metastatic melanoma. Cancer Biol Ther. 2005;4:1318–24.
Allen BJ, Singla AA, Rizvi SMA, Graham P, Bruchertseifer F, Apostolidis C, et al. Analysis of patient survival in a phase I trial of systemic targeted α-therapy for metastatic melanoma. Immunotherapy. 2011;3:1041–50.
Raja C, Graham P, Abbas Rizvi SM, Song E, Goldsmith H, Thompson J, et al. Interim analysis of toxicity and response in phase 1 trial of systemic targeted alpha therapy for metastatic melanoma. Cancer Biol Ther. 2007;6:846–52.
Rosenblat TL, McDevitt MR, Mulford DA, Pandit-Taskar N, Divgi CR, Panageas KS, et al. Sequential cytarabine and alpha-particle immunotherapy with bismuth-213-lintuzumab (HuM195) for acute myeloid leukemia. Clin Cancer Res. 2010;16:5303–11.
Jurcic JG, Caron PC, Nikula TK, Papadopoulos EB, Finn RD, Gansow OA, et al. Radiolabeled anti-CD33 monoclonal antibody M195 for myeloid leukemias. Cancer Res. 1995;55:5908s–10s.
Autenrieth ME, Seidl C, Bruchertseifer F, Horn T, Kurtz F, Feuerecker B, et al. Treatment of carcinoma in situ of the urinary bladder with an alpha-emitter immunoconjugate targeting the epidermal growth factor receptor: a pilot study. Eur J Nucl Med Mol Imaging. 2018;45:1364–71.
Kratochwil C, Giesel FL, Bruchertseifer F, Mier W, Apostolidis C, Boll R, et al. 213Bi-DOTATOC receptor-targeted alpha-radionuclide therapy induces remission in neuroendocrine tumours refractory to beta radiation: a first-in-human experience. Eur J Nucl Med Mol Imaging. 2014;41:2106–19.
Krolicki L, Bruchertseifer F, Kunikowska J, Koziara H, Królicki B, Jakuciński M, et al. Prolonged survival in secondary glioblastoma following local injection of targeted alpha therapy with 213Bi-substance P analogue. Eur J Nucl Med Mol Imaging. 2018;45:1636–44.
Krolicki L, Bruchertseifer F, Kunikowska J, Koziara H, Krolici B, Al E. Safety and efficacy of targeted alpha therapy with 213Bi-DOTA-substance P in recurrent glioblastoma. Eur J Nucl Med Mol Imaging. 2019;46:614–22.
Cordier D, Forrer F, Bruchertseifer F, Morgenstern A, Apostolidis C, Good S, et al. Targeted alpha-radionuclide therapy of functionally critically located gliomas with 213Bi-DOTA-[Thi8,Met(O2)11]-substance P: a pilot trial. Eur J Nucl Med Mol Imaging. 2010;37:1335–44.
Meredith RF, Torgue J, Azure MT, Shen S, Saddekni S, Banaga E, et al. Pharmacokinetics and imaging of 212 Pb-TCMC-trastuzumab after intraperitoneal administration in ovarian cancer patients. Cancer Biother Radiopharm. 2014;29:12–7.
Meredith R, Torgue J, Shen S, Fisher DR, Banaga E, Bunch P, et al. Dose escalation and dosimetry of first-in-human α radioimmunotherapy with 212Pb-TCMC-trastuzumab. J Nucl Med. 2014;55:1636–42.
Poeppel TD, Handkiewicz-Junak D, Andreeff M, Becherer A, Bockisch A, Fricke E, et al. EANM guideline for radionuclide therapy with radium-223 of metastatic castration-resistant prostate cancer. Eur J Nucl Med Mol Imaging. 2018;45:824–45.
Du Y, Carrio I, De Vincentis G, Fanti S, Ilhan H, Mommsen C, et al. Practical recommendations for radium-223 treatment of metastatic castration-resistant prostate cancer. Eur J Nucl Med Mol Imaging. 2017;44:1671–8.
National Institute for Health and Care Excellence. Radium-223 dichloride for treating hormone-relapsed prostate cancer with bone metastases (TA412). 2016. p. 1–41. Available from: https://www.nice.org.uk/guidance/ta412. Accessed 20 Dec 2016.
FierceBiotech. J&J leads $25M Series A in targeted radiotherapy startup. 2017. Available from: https://www.fiercebiotech.com/venture-capital/j-j-leads-25m-series-a-targeted-radiotherapy-startup. Accessed 20 Feb 2019.
Acknowledgements
Medical writing support was provided by AS&K Communications and funded by Bayer.
Availability of data and material
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.
Funding
Preparation of this manuscript was funded by Bayer.
Author information
Authors and Affiliations
Contributions
All authors were involved in the analysis and interpretation of the review results. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
Sabina Dizdarevic has served as a consultant to Bayer and has received travel support, and educational grants from Bayer, and has received honoraria from GE Healthcare.
Ralph McCready declares no conflict of interest in relation to this article.
Sobhan Vinjamuri was a member of an Advisory Board for Bayer and has received honoraria from Ipsen and Advanced Accelerator Applications.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Oncology – Genitourinary
Rights and permissions
About this article
Cite this article
Dizdarevic, S., McCready, R. & Vinjamuri, S. Radium-223 dichloride in prostate cancer: proof of principle for the use of targeted alpha treatment in clinical practice. Eur J Nucl Med Mol Imaging 47, 192–217 (2020). https://doi.org/10.1007/s00259-019-04475-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-019-04475-5