Abstract
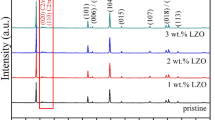
Li2ZrO3-modified LiNi0.5Mn0.5O2 materials with improved electrochemical performance were directly synthesized by a simple mechanical milling route with ZrO2, Li2CO3 and Ni0.5Mn0.5(OH)2 precursors and a high temperature calcination in air atmosphere. The influences of ZrO2 contents on the microstructures and electrochemical properties of LiNi0.5Mn0.5O2 electrode materials were investigated through X-Ray diffraction, scanning electron microscope, energy dispersive spectroscopy and electrochemical tests. The results showed that ZrO2 can be completely converted into Li2ZrO3 in the form of a coating layer covering the surface of LiNi0.5Mn0.5O2 after a heat treatment process. Li2ZrO3 coating can be formed and dispersed homogenously on the surface of 1 mol% Li2ZrO3-modified LiNi0.5Mn0.5O2 materials. The electrochemical tests confirmed 1 mol% Li2ZrO3-modified LiNi0.5Mn0.5O2 materials exhibited the best discharge capacity of 158.3 mAh g−1 after 100 cycles between 2.75 and 4.35 V at 0.2 C, with an excellent capacity retention of 97.2% and higher discharge capacity at −20 °C than that of the pristine LiNi0.5Mn0.5O2. The enhanced cycling stability and low temperature performance may be attributed to the remarkable synergistic effects of Li2ZrO3 protective layer and its homogeneous distribution on LiNi0.5Mn0.5O2 surface with low Li/Ni cation mixing, high electric conductivity and good structure stability.








Similar content being viewed by others
References
P. Simon, Y. Gogotsi, B. Dunn, Science 343, 1210–1211 (2014)
Z.Q. Yan, W.L. Yao, L. Hu, D.D. Liu, C.D. Wang, C.S. Lee, Nanoscale 7, 5563–5577 (2015)
Y. Yang, S. Li, Q. Zhang, Y. Zhang, S. Xu, Ind. Eng. Chem. Res. 56, 175–182 (2017)
T.E. Quine, M.J. Duncan, A.R. Armstrong, A.D. Robertson, P.G. Bruce, J. Mater. Chem. 10, 2838–2841 (2007)
Y. Hinuma, Y.S. Meng, K. Kang, G. Ceder, Chem. Mater. 19, 1790–1800 (2007)
X.L. Meng, S.M. Dou, W.L. Wang, J. Power Sources 184, 489–493 (2008)
M. Yoncheva, R. Stoyanova, E. Zhecheva, R. Alcantara, J.L. Tirado, J. Alloys Compd. 475, 96–101 (2009)
K. Sakamoto, M. Hirayama, H. Konishi, N. Sonoyama, N. Dupre, D. Guyomard, K. Tamura, J. Mizuki, R. Kanno, Phys. Chem. Chem. Phys. 12, 3815–3823 (2010)
Y.M. Liu, B.L. Chen, F. Cao, X. Zhao, J. Yuan, J. Mater. Chem. 21, 10437–10441 (2011)
F. Li, G. Yang, G. Jia, X. Shangguan, Q. Zhuge, B. Bai, J. Appl. Electrochem. 47, 1189–1201 (2017)
Y. Ding, D. Mu, B. Wu, R. Wang, Z. Zhao, F. Wu, Appl. Energy 195, 586–599 (2017)
H. Kobayashi, H. Sakaebe, H. Kageyama, K. Tatsumi, Y. Arachi, J. Mater. Chem. 13, 590–595 (2003)
Z. Wang, E. Liu, L. Guo, C. Shi, C. He, J. Li, N. Zhao, Surf. Coat. Technol. 235, 570–576 (2013)
J.Z. Kong, C. Ren, G.A. Tai, X. Zhang, A.D. Li, D. Wu, H. Li, F. Zhou, J. Power Sources 266, 433–439 (2014)
J. Cho, J.K. Yong, B. Park, Chem. Mater. 32, 3788–3791 (2000)
W. Feng, W. Meng, Y. Su, C. Shi, B. Xu, J. Power Sources 191, 628–632 (2009)
H. Liu, G.X. Wang, D. Wexler, J.Z. Wang, H.K. Liu, Electrochem. Commun. 10, 165–169 (2008)
J.Q. Zhao, Y. Wang, Nano Energy 2, 882–889 (2013)
H. Han, F. Qiu, Z. Liu, X. Han, Ceram. Int. 41, 8779–8784 (2015)
E. Jung, Y.J. Park, J. Electroceram. 29, 23–28 (2012)
B.J. Hwang, S.K. Hu, C.H. Chen, C.Y. Chen, H.S. Sheu, J. Power Sources 174, 61–765 (2007)
S.K. Hu, G.H. Cheng, M.Y. Cheng, B.J. Hwang, R. Santhanam, J. Power Sources 188, 564–569 (2009)
J.Z. Kong, S.S. Wang, G.A. Tai, L. Zhu, L.G. Wang, H.F. Zhai, D. Wu, A.D. Li, H. Li, J. Alloys Compd. 657, 593–600 (2016)
L. Li, Z. Chen, Q. Zhang, M. Xu, X. Zhou, H. Zhu, K. Zhang, J. Mater. Chem. A 3, 894–904 (2015)
W. Wang, Z. Yin, Z. Wang, X. Li, H. Guo, D. Wang, J. Alloys Compd. 646, 454–460 (2015)
J. Wang, Y. Yu, B. Li, T. Fu, D. Xie, J. Cai, J. Zhao, Phys. Chem. Chem. Phys. 17, 32033–32043 (2015)
W. Wang, Z. Yin, J. Wang, Z. Wang, X. Li, H. Guo, J. Alloys Compd. 651, 737–743 (2015)
S.B. Lim, H. Lee, Y.J. Park, J. Electroceram. 37, 92–97 (2016)
C. Wang, L. Chen, H. Zhang, Y. Yang, F. Wang, F. Yin, G. Yang, Electrochim. Acta 119, 236–242 (2014)
D. Wang, X. Li, Z. Wang, H. Guo, Z. Huang, L. Kong, J. Ru, J. Alloys Compd. 647, 612–619 (2015)
Y. Xu, Y. Liu, Z. Lu, H. Wang, D. Sun, G. Yang, Appl. Surf. Sci. 361, 150–156 (2016)
S. Zhong, P. Chen, W. Yao, ECS Electrochem. Lett. 4, A45–A48 (2015)
S. Zhong, M. Lai, W. Yao, Z. Li, Electrochim. Acta 212, 343–351 (2016)
H.Q. Liu, Y.M. Hu, Y.B. Li, H.S. Gu, Mater. Chem. Phys. 138, 440–443 (2013)
H. Gwon, S.W. Kim, Y.U. Park, J. Hong, G. Ceder, S. Jeon, K. Kang, Inorg. Chem. 53, 8083–8087 (2014)
T. Ohzuku, A. Ueda, M. Nagayama, J. Electrochem. Soc. 140, 1862–1870 (1993)
A.M.A. Hashem, A.E. Abdel-Ghany, A.E. Eid, J. Trottier, K. Zaghib, J. Power Sources 196, 8632–8637 (2011)
X. Zhang, A. Mauger, L. Qi, H. Groult, L. Perrigaud, Electrochim. Acta 55, 6440–6449 (2010)
S. Jouanneau, K.W. Eberman, L.J. Krause, J.R. Dahn, J. Electrochem. Soc. 150, A1637–A1642 (2003)
S.N. Lim, W. Ahn, S.H. Yeon, S.B. Park, Electrochim. Acta 136, 1–9 (2014)
F. Nobili, S. Dsoke, M. Minicucci, F. Croce, R. Marassi, J. Phys. Chem. B 110, 11310–11313 (2006)
X.Y. Qiu, Q.C. Zhuang, Q.Q. Zhang, R. Cao, Y.H. Qiang, P.Z. Ying, S.G. Sun, J. Electroanal. Chem. 687, 35–44 (2012)
W. Yao, Y. Liu, Q. Dai, D. Li, Y. Yu, Q. Jing, J. Chin. Chem. Soc. 64, 539–546 (2017)
C.H. Liang, L.B. Liu, Z. Jia, C. Dai, Y. Xiong, Electrochim. Acta 186, 413–419 (2015)
W. Yao, Q. Dai, Y. Liu, Q. Zhang, S. Zhong, Z. Yan, ChemElectroChem 4, 1236–1242 (2017)
Acknowledgments
The authors are grateful for the financial support of this work by National Natural Science Fund of China (No.51372104), Jiangxi Province Science and Technology Plan Project (No.20141BBE50019), Foundation of Jiangxi Educational Committee (No. GJJ160601), Finance and Education Plan of Ganzhou City (No.197[2017]) and Doctoral scientific research foundation of Jiangxi University of Science and Technology (No. jxxjbs16025).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Fig. S1
SEM image and the corresponding element mapping of O, Ni, Mn, Zr and EDS images of 1-LZO@LNMO material. (JPG 443 kb)
Fig. S2
SEM image and the corresponding element mapping of Zr for LZO-modified LiNi0.5Mn0.5O2 materials. (JPG 365 kb)
Fig. S3
Rate capability of LZO-modified LiNi0.5Mn0.5O2 materials from 0.2C to 5C between 2.75 and 4.35 V. (JPG 119 kb)
Rights and permissions
About this article
Cite this article
Yao, W., Zhang, H., Zhong, S. et al. Facile synthesis of Li2ZrO3-modified LiNi0.5Mn0.5O2 cathode material from a mechanical milling route for lithium-ion batteries. J Electroceram 43, 84–91 (2019). https://doi.org/10.1007/s10832-018-0158-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10832-018-0158-6