Abstract
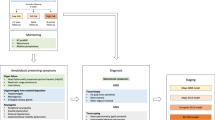
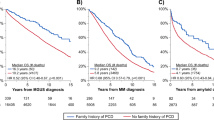
This study evaluates newly diagnosed IgM (6%, n = 75/1174) vs. non-IgM light chain amyloidosis patients. IgM amyloid patients had lower light chains (12.5 vs. 22.5 mg/dL; p < 0.001). Heart (56% vs. 73%, p = 0.002) and >1 organ involvement (31% vs. 44%, p = 0.02) was less common in IgM amyloidosis, while soft tissue and peripheral nerve involvement was more common. t(11;14) was less common (27% vs. 50%, p = 0.008) in IgM amyloidosis. Rates of MYD88L265P and CXCR4WHIM mutation in IgM amyloidosis were 58% (29/50) and 17% (8/46). Diagnosis after hematopathology review in IgM amyloidosis was pure plasma cell neoplasm (PPCN) in 23% (16/70), lymphoplasmacytic neoplasm (LPL) in 63% (44/70) patients, and other (14%). LPL vs. PPCN groups had distinct genetic abnormalities: t(11;14): 0% (0/18) vs. 60% (9/15), p < 0.001; MYD88L265P mutation: 84% (27/32) vs. 0% (0/14), p < 0.001; CXCR4 mutation: 29% (8/28) vs. 0% (0/14), p = 0.04. Overall survival was shorter in IgM AL when stratified by Mayo 2012 stage; stage 1/2 (59 vs. 125.9 months, p = 0.003) and stage 3/4 (6.5 vs. 12.9 months, p = 0.075), likely due to lower hematologic response rates (6 months: 39% vs. 59%, p = 0.008). We characterized two subtypes of IgM amyloidosis (LPL/PPCN). This can aid in therapeutic decision-making, with treatment directed at the clonal disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Gertz MA, Kyle RA, Noel P. Primary systemic amyloidosis: a rare complication of immunoglobulin M monoclonal gammopathies and Waldenstrom’s macroglobulinemia. J Clin Oncol. 1993;11:914–20.
Gertz MA, Buadi FK, Hayman SR. IgM amyloidosis: clinical features in therapeutic outcomes. Clin Lymphoma Myeloma Leuk. 2011;11:146–8.
Palladini G, Russo P, Bosoni T, Sarais G, Lavatelli F, Foli A, et al. AL amyloidosis associated with IgM monoclonal protein: a distinct clinical entity. Clin Lymphoma Myeloma. 2009;9:80–3.
Wechalekar AD, Lachmann HJ, Goodman HJ, Bradwell A, Hawkins PN, Gillmore JD. AL amyloidosis associated with IgM paraproteinemia: clinical profile and treatment outcome. Blood. 2008;112:4009–16.
Zanwar S, Abeykoon JP, Ansell SM, Gertz MA, Dispenzieri A, Muchtar E, et al. Primary systemic amyloidosis in patients with Waldenstrom macroglobulinemia. Leukemia. 2019;33:790–4.
Milani P, Merlini G. Monoclonal IgM-related AL amyloidosis. Best Pract Res Clin Haematol. 2016;29:241–8.
Terrier B, Jaccard A, Harousseau JL, Delarue R, Tournilhac O, Hunault-Berger M, et al. The clinical spectrum of IgM-related amyloidosis: a French nationwide retrospective study of 72 patients. Medicine. 2008;87:99–109.
Sissoko M, Sanchorawala V, Seldin D, Sworder B, Angelino K, Broce M, et al. Clinical presentation and treatment responses in IgM-related AL amyloidosis. Amyloid. 2015;22:229–35.
Sachchithanantham S, Roussel M, Palladini G, Klersy C, Mahmood S, Venner CP, et al. European Collaborative Study defining clinical profile outcomes and novel prognostic criteria in monoclonal immunoglobulin M-related light chain amyloidosis. J Clin Oncol. 2016;34:2037–45.
Muchtar E, Dispenzieri A, Kumar SK, Ketterling RP, Dingli D, Lacy MQ, et al. Interphase fluorescence in situ hybridization in untreated AL amyloidosis has an independent prognostic impact by abnormality type and treatment category. Leukemia. 2017;31:1562–9.
Bochtler T, Hegenbart U, Kunz C, Granzow M, Benner A, Seckinger A, et al. Translocation t(11;14) is associated with adverse outcome in patients with newly diagnosed AL amyloidosis when treated with bortezomib-based regimens. J Clin Oncol. 2015;33:1371–8.
Treon SP, Cao Y, Xu L, Yang G, Liu X, Hunter ZR. Somatic mutations in MYD88 and CXCR4 are determinants of clinical presentation and overall survival in Waldenstrom macroglobulinemia. Blood. 2014;123:2791–6.
Treon SP, Tripsas CK, Meid K, Warren D, Varma G, Green R, et al. Ibrutinib in previously treated Waldenstrom’s macroglobulinemia. N. Engl J Med. 2015;372:1430–40.
Chakraborty R, Novak AJ, Ansell SM, Muchtar E, Kapoor P, Hayman SR, et al. First report of MYD88 L265P somatic mutation in IgM-associated light-chain amyloidosis. Blood. 2016;127:2936–8.
Thakral B, Kanagal-Shamanna R, Systemic AL. amyloidosis associated with Waldenstrom macroglobulinemia: an unusual presenting complication. Blood. 2016;127:168.
Gertz MA, Comenzo R, Falk RH, Fermand JP, Hazenberg BP, Hawkins PN, et al. Definition of organ involvement and treatment response in immunoglobulin light chain amyloidosis (AL): a consensus opinion from the 10th International Symposium on Amyloid and Amyloidosis, Tours, France, 18-22 April 2004. Am J Hematol. 2005;79:319–28.
Palladini G, Dispenzieri A, Gertz MA, Kumar S, Wechalekar A, Hawkins PN, et al. New criteria for response to treatment in immunoglobulin light chain amyloidosis based on free light chain measurement and cardiac biomarkers: impact on survival outcomes. J Clin Oncol. 2012;30:4541–9.
Palladini G, Hegenbart U, Milani P, Kimmich C, Foli A, Ho AD, et al. A staging system for renal outcome and early markers of renal response to chemotherapy in AL amyloidosis. Blood. 2014;124:2325–32.
Milani P, Basset M, Russo F, Foli A, Merlini G, Palladini G. Patients with light-chain amyloidosis and low free light-chain burden have distinct clinical features and outcome. Blood. 2017;130:625–31.
Dittrich T, Bochtler T, Kimmich C, Becker N, Jauch A, Goldschmidt H, et al. AL amyloidosis patients with low amyloidogenic free light chain levels at first diagnosis have an excellent prognosis. Blood. 2017;130:632–42.
Sidana S, Tandon N, Dispenzieri A, Gertz MA, Buadi FK, Lacy MQ, et al. Clinical presentation and outcomes in light chain amyloidosis patients with non-evaluable serum free light chains. Leukemia. 2018;32:729–35.
Owen RG, Kyle RA, Stone MJ, Rawstron AC, Leblond V, Merlini G, et al. Response assessment in Waldenstrom macroglobulinaemia: update from the VIth International Workshop. Br J Haematol. 2013;160:171–6.
Rosado FG, Morice WG, He R, Howard MT, Timm M, McPhail ED. Immunophenotypic features by multiparameter flow cytometry can help distinguish low grade B-cell lymphomas with plasmacytic differentiation from plasma cell proliferative disorders with an unrelated clonal B-cell process. Br J Haematol. 2015;169:368–76.
Morice WG, Chen D, Kurtin PJ, Hanson CA, McPhail ED. Novel immunophenotypic features of marrow lymphoplasmacytic lymphoma and correlation with Waldenstrom’s macroglobulinemia. Mod Pathol. 2009;22:807–16.
Shi M, Ternus JA, Ketterling RP, Jevremovic D, McPhail ED. Immunophenotypic and laboratory features of t(11;14)(q13;q32)-positive plasma cell neoplasms. Leuk Lymphoma. 2018;59:1913–9.
Fonseca R, Blood E, Rue M, Harrington D, Oken MM, Kyle RA, et al. Clinical and biologic implications of recurrent genomic aberrations in myeloma. Blood. 2003;101:4569–75.
Binder M, Rajkumar SV, Ketterling RP, Dispenzieri A, Lacy MQ, Gertz MA, et al. Occurrence and prognostic significance of cytogenetic evolution in patients with multiple myeloma. Blood Cancer J. 2016;6:e401.
Vrana JA, Gamez JD, Madden BJ, Theis JD, Bergen HR 3rd, Dogan A. Classification of amyloidosis by laser microdissection and mass spectrometry-based proteomic analysis in clinical biopsy specimens. Blood. 2009;114:4957–9.
Pika T, Hegenbart U, Flodrova P, Maier B, Kimmich C, Schonland SO. First report of ibrutinib in IgM-related amyloidosis: few responses, poor tolerability, and short survival. Blood. 2018;131:368–71.
Treon SP, Xu L, Yang G, Zhou Y, Liu X, Cao Y, et al. MYD88 L265P somatic mutation in Waldenstrom’s macroglobulinemia. N. Engl J Med. 2012;367:826–33.
Sidana S, Tandon N, Dispenzieri A, Gertz MA, Buadi F, Lacy M, et al. Factors predicting organ response in light chain amyloidosis (AL). J Clin Oncol. 2017;35 15_suppl:8048.
Muchtar E, Dispenzieri A, Leung N, Lacy MQ, Buadi FK, Dingli D, et al. Optimizing deep response assessment for AL amyloidosis using involved free light chain level at end of therapy: failure of the serum free light chain ratio. Leukemia. 2019;33:527–31.
Fowler N, Kahl BS, Lee P, Matous JV, Cashen AF, Jacobs SA, et al. Bortezomib, bendamustine, and rituximab in patients with relapsed or refractory follicular lymphoma: the phase II VERTICAL study. J Clin Oncol. 2011;29:3389–95.
Flinn IW, Thompson DS, Boccia RV, Miletello G, Lipman A, Flora D, et al. Bendamustine, bortezomib and rituximab produces durable complete remissions in patients with previously untreated, low grade lymphoma. Br J Haematol. 2018;180:365–73.
Sidiqi MH, Buadi FK, Dispenzieri A, Warsame R, Lacy MQ, Dingli D, et al. Autologous stem cell transplant for IgM-associated amyloid light-chain amyloidosis. Biol Blood Marrow Transplant. 2019;25:e108–11.
Gertz MA, Hayman SR, Buadi FK. Transplantation for IgM amyloidosis and IgM myeloma. Clin Lymphoma Myeloma. 2009;9:77–9.
Marzolini MA, Thomson KJ, Dorman J, D’Sa S. BEAM-conditioned autologous SCT improves the quality of response in Waldenstrom’s macroglobulinaemia and lymphoplasmacytic lymphoma: a single centre’s 10-year experience. Bone Marrow Transplant. 2014;49:1231–2.
Kyriakou C, Canals C, Sibon D, Cahn JY, Kazmi M, Arcese W, et al. High-dose therapy and autologous stem-cell transplantation in Waldenstrom macroglobulinemia: the Lymphoma Working Party of the European Group for Blood and Marrow Transplantation. J Clin Oncol. 2010;28:2227–32.
Acknowledgements
We would like to acknowledge Ms Kim Henderson and Ms Katie Halverson for their assistance in testing stored biospecimens.
Funding
Katherine McCleary Research Fund and K. Edward Jacobi Research Partners Fund of the International Waldenström’s Macroglobulinemia Foundation; Amyloidosis Foundation.
Author information
Authors and Affiliations
Contributions
SS and MAG designed the study, collected the data, performed the analysis, and wrote the first draft of the manuscript. RLK and DL reviewed and analyzed the hematopathology data. PTG supervised FISH testing on cryopreserved bone marrow aspirates. RH supervised MYD88 and CXCR4 mutation testing on cryopreserved bone marrow aspirates. EDM supervised mass spectrometry testing on archival samples. EDM and SD reviewed mass spectrometry testing. All authors critically reviewed the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
SS: Consultancy: Janssen. AD: research funding from Celgene, Takeda, Prothena, Janssen, Pfizer, Alnylam, and GSK. PK: research funding from Celgene and Takeda. MQL: research funding from Celgene. SKK: research funding and membership on an entity’s Board of Directors or advisory committees: AbbVie, Celgene, Janssen, KITE, and Merck. Membership on an entity’s Board of Directors or advisory committees: Oncopeptides and Takeda. Research funding from Novartis and Roche. MAG: honoraria/consultancy from Ionis, Alnylam, Prothena, Celgene, Janssen, Spectrum, Annexon, Apellis, Amgen, Medscape, Abbvie, Research to Practice, Physicians Education Resource, and Teva. The other authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Sidana, S., Larson, D.P., Greipp, P.T. et al. IgM AL amyloidosis: delineating disease biology and outcomes with clinical, genomic and bone marrow morphological features. Leukemia 34, 1373–1382 (2020). https://doi.org/10.1038/s41375-019-0667-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41375-019-0667-6
This article is cited by
-
A retrospective analysis of clinical features and treatment outcome in 21 patients with immunoglobulin M-related light-chain amyloidosis in Japan: a study from the Amyloidosis Research Committee
International Journal of Hematology (2023)
-
Kidney injury and disease in patients with haematological malignancies
Nature Reviews Nephrology (2021)
-
Marked progress in AL amyloidosis survival: a 40-year longitudinal natural history study
Blood Cancer Journal (2021)
-
Minimal residual disease negativity by next-generation flow cytometry is associated with improved organ response in AL amyloidosis
Blood Cancer Journal (2021)