Abstract
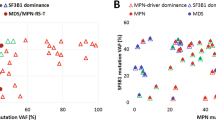
Besides histopathological findings there are no indicators of increased risk for fibrotic progression in myeloproliferative neoplasms (MPN). Age-related clonal hematopoiesis (ARCH/CHIP) is a frequent finding in the elderly and combinations with MPN driver mutations (JAK2, MPL, and CALR) have been described. To determine the impact of ARCH/CHIP-related mutations for development of fibrosis in primary myelofibrosis (PMF), the mutational status of cases with fibrotic progression from grade 0 to grade 2/3 (n = 77) as evidenced by follow-up bone marrow biopsies (median 6.2 years) was compared with prefibrotic PMF samples without development of fibrosis (n = 27; median follow-up 7.3 years). Frequent ARCH/CHIP-associated mutations (TET2, ASXL1, and DNMT3A) demonstrable at presentation were not connected with fibrotic progression. However, mutations which are rarely found in ARCH/CHIP (SRSF2, U2AF1, SF3B1, IDH1/2, and EZH2) were present in 24.7% of cases with later development of fibrosis and not detectable in cases staying free from fibrosis (P = 0.0028). Determination of the tumor mutational burden (TMB) in a subgroup of cases (n = 32) did not show significant differences (7.68 mutations/MB vs. 6.85 mutations/MB). We conclude that mutations rarely found in ARCH/CHIP provide an independent risk factor for rapid fibrotic progression (median 2.0 years) when manifest already at first presentation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Arber D, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, et al. The 2016 revision of the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391–405.
Czader M, Orazi A. Acute myeloid leukemia and other types of disease progression in myeloproliferative neoplasms. Am J Clin Pathol. 2015;144:188–206.
Latagliata R, Polverelli N, Tieghi A, Palumbo GA, Breccia M, Sabattini E, et al. Comparison of JAK2V617F -positive essential thrombocythaemia and early primary myelofibrosis: the impact of mutation burden and histology. Hematol Oncol. 2018;36:269–75.
Hernández-Boluda JC, Pereira A, Correa JG, Alvarez-Larrán A, Ferrer-Marín F, Raja JM, et al. Prognostic risk models for transplant decision-making in myelofibrosis. Ann Hematol. 2018;97:813–20.
Tefferi A, Guglielmelli P, Nicolosi M, Mannelli F, Mudireddy M, Bartalucci N, et al. GIPSS: genetically inspired prognostic scoring system for primary myelofibrosis. Leukemia. 2018;32:1631–42.
Lehmann U, Bartels S, Hasemeier B, Geffers R, Schlue J, Büsche G, et al. SRSF2 mutation is present in the hypercellular and prefibrotic stage of primary myelofibrosis. Blood. 2013;121:4011–2.
Bartels S, Lehmann U, Büsche G, Schlue J, Mozer M, Stadler J, et al. SRSF2 and U2AF1 mutations in primary myelofibrosis are associated with JAK2 and MPL but not calreticulin mutation and may independently reoccur after allogeneic stem cell transplantation. Leukemia. 2015;29:253–5.
Bartels S, Faisal M, Büsche G, Schlue J, Kreipe H, Lehmann U. Fibrotic progression in Polycythemia vera is associated with early concomitant driver-mutations besides JAK2. Leukemia. 2018;32:556–8.
Busque L, Patel JP, Figueroa ME, Vasanthakumar A, Provost S, Hamilou Z, et al. Recurrent somatic TET2 mutations in normal elderly individuals with clonal hematopoiesis. Nat Genet. 2012;44:1179–81.
Shlush LI, Zandi S, Itzkovitz S, Schuh AC. Aging, clonal hematopoiesis and preleukemia: not just bad luck? Int J Hematol. 2015;102:513–22.
Steensma DP, Bejar R, Jaiswal S, Lindsley RC, Sekeres MA, Hasserjian RP, et al. Clonal hematopoiesis of indeterminate potential and its distinction from myelodysplastic syndromes. Blood. 2015;126:9–16.
Steensma DP. Clinical consequences of clonal hematopoiesis of indeterminate potential. Blood Adv. 2018;2:3404–10.
Kreipe H, Jaquet K, Felgner J, Radzun HJ, Parwaresch MR. Clonal granulocytes and bone marrow cells in the cellular phase of agnogenic myeloid metaplasia. Blood. 1991;78:1814–7.
Kvasnicka HM, Beham-Schmid C, Bob R, Dirnhofer S, Hussein K, Kreipe H, et al. Problems and pitfalls in grading of bone marrow fibrosis, collagen deposition and osteosclerosis - a consensus-based study. Histopathology. 2016;68:905–15.
Faisal M, Stark H, Büsche G, Schlue J, Teiken K, Kreipe HH, et al. Comprehensive mutation profiling and mRNA expression analysis in atypical chronic myeloid leukemia in comparison with chronic myelomonocytic leukemia. Cancer Med. 2019;8:742–50.
Wang K, Li M, Hakonarson H. ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acid Res. 2010;38:e164.
Buhr T, Hebeda K, Kaloutsi V, Porwit A, Van der Walt J, Kreipe H. European bone marrow working group trial on reproducibility of World Health Organization criteria to discriminate essential thrombocythemia from prefibrotic primary myelofibrosis. Haematologica. 2012;97:360–5.
Gianelli U, Bossi A, Cortinovis I, Sabattini E, Tripodo C, Boveri E, et al. Reproducibility of the WHO histological criteria for the diagnosis of Philadelphia chromosomenegative myeloproliferative neoplasms. Mod Pathol. 2014;27:814–22.
Vannucchi AM, Harrison CN. Emerging treatments for classical myeloproliferative neoplasms. Blood. 2017;129:693–703.
Gibson CJ, Steensma DP. New insights from studies of clonal hematopoiesis. Clin Cancer Res. 2018;24:4633–42.
Ortmann CA, Kent DG, Nangalia J, Silber Y, Wedge DC, Grinfeld J, et al. Effect of mutation order on myeloproliferative neoplasms. N. Engl J Med. 2015;372:601–12.
Lundberg P, Karow A, Nienhold R, Looser R, Hao-Shen H, Nissen I, et al. Clonal evolution and clinical correlates of somatic mutations in myeloproliferative neoplasms. Blood. 2014;123:2220–8.
Link DC, Walter MJ. ‘CHIP’ping away at clonal hematopoiesis. Leukemia. 2016;30:1633–5.
Zhang L, McGraw KL, Sallman DA, List AF. The role of p53 in myelodysplastic syndromes and acute myeloid leukemia: molecular aspects and clinical implications. Leuk Lymphoma. 2017;58:1777–90.
Passamonti F, Rumi E, Pietra D, Elena C, Boveri E, Arcaini L, et al. A prospective study of 338 patients with polycythemia vera: the impact of JAK2 (V617F) allele burden and leukocytosis on fibrotic or leukemic disease transformation and vascular complications. Leukemia. 2010;24:1574–9.
Allgäuer M, Budczies J, Christopoulos P, Endris V, Lier A, Remlep E, et al. Implementing tumor mutational burden (TMB) analysis in routine diagnostics-a primer for molecular pathologists and clinicians. Transl Lung Cancer Res. 2018;7:703–15.
Author information
Authors and Affiliations
Contributions
SB, UL, and HK planned the study, interpreted data, and wrote the paper; SB, MF, BH, ES, JV, and LW performed experiments and analyzed the data; GB, JS, and HK contributed the histopathological diagnosis of the patient samples.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bartels, S., Faisal, M., Büsche, G. et al. Mutations associated with age-related clonal hematopoiesis in PMF patients with rapid progression to myelofibrosis. Leukemia 34, 1364–1372 (2020). https://doi.org/10.1038/s41375-019-0668-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41375-019-0668-5
This article is cited by
-
Primäre Myelofibrose
Die Onkologie (2023)
-
SRSF2-P95H decreases JAK/STAT signaling in hematopoietic cells and delays myelofibrosis development in mice
Leukemia (2023)
-
Next-generation sequencing redefines the diagnosis of triple-negative myeloproliferative neoplasms
Annals of Hematology (2022)
-
Genome-wide DNA methylation profiling is able to identify prefibrotic PMF cases at risk for progression to myelofibrosis
Clinical Epigenetics (2021)
-
Knochenmarkfibrose bei primärer Myelofibrose in Abhängigkeit von myelodysplasie- und altersassoziierten Mutationen der Hämatopoese
Der Pathologe (2020)