Abstract
Microbial electrosynthesis (MES) is potentially useful for the biological conversion of carbon dioxide into value-added chemicals and biofuels. The study evaluated several limiting factors that affect MES performance. Among all these factors, the optimization of the applied cell voltage, electrode spacing, and trace elements in catholytes may significantly improve the MES performance. MES was operated under the optimal condition with an applied cell voltage of 3 V, an electrode spacing of 8 cm, 2× salt solution, and 8× trace element of catholyte for 100 days, and the maximum acetate concentration reached 7.8 g L−1. The microbial community analyses of the cathode chamber over time showed that Acetobacterium, Enterobacteriaceae, Arcobacter, Sulfurospirillum, and Thioclava were the predominant genera during the entire MES process. The abundance of Acetobacterium first increased and then decreased, which was consistent with that of acetate production. These results provided useful hints for replacing the potentiostatic control of the cathodes in the future construction and operation of MES. Such results might also contribute to the practical operation of MES in large-scale systems.

Similar content being viewed by others
Introduction
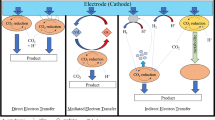
The current worldwide emission of greenhouse gases, such as CO2, is mainly attributed to the use of fossil fuels (O’Neill and Oppenheimer 2002). Researchers should develop the cost-effective CO2 transformation technologies to balance the increasing energy demand with the need to economically reduce CO2 emissions. Microbial electrosynthesis (MES) is a novel approach to convert CO2 to value-added chemicals with renewable electricity as an energy source (Lovley 2011; Nevin et al. 2010; Rabaey and Rozendal 2010). In MES, electroautotrophic microbes can utilize electrons directly or indirectly through H2 from a cathode to reduce CO2 to organic value-added chemicals (Lovley and Nevin 2013; Shin et al. 2017).
Since Nevin et al. (2010) first demonstrated the proof-of-concept of MES, many crucial elements, such as electrode materials (Bajracharya et al. 2015; Jourdin et al. 2015; Marshall et al. 2013; Song et al. 2018), reactor designs (Giddings et al. 2015), membranes (Gildemyn et al. 2017; Xiang et al. 2017), microbial communities (Nevin et al. 2011; Patil et al. 2015; Modestra and Mohan 2017), and product separation (Bajracharya et al. 2017; Batlle-Vilanova et al. 2017; Gildemyn et al. 2015), have been studied in MES. In comparison with pure strains (Nie et al. 2013; Nevin et al. 2010; Zhang et al. 2013), mixed culture (Dong et al. 2018; Marshall et al. 2013; Modestra and Mohan 2017; Jourdin et al. 2014; Patil et al. 2015; Song et al. 2017, 2018) is possibly more suitable for the commercialization of MES because it has better resistance to environmental disturbances, high biomass, and acetate production rate (Marshall et al. 2013). The production and investment costs of producing acetate by MES (Christodoulou and Velasquez-Orta 2016) were relatively high (1.44 £ kg−1, 1770 £ t−1). Therefore, it is necessary to increase the productivity of acetate further and reduce costs in order to advance toward industrialization. In addition, the bio-production rate of acetate should reach at least 4550 mol per day to achieve economic feasibility. This analysis indirectly indicates that MES can be scaled up as high-value chemicals producers.
MES systems need electrical energy to supply electrons for CO2 bioreduction. These electrical energies may come from solar and wind sources; thus, the utilization of electricity from renewable sources for CO2 reduction in MES can alleviate existing challenges associated with the intermittent nature of renewable energy by storing electricity in a chemical form (Lovley and Nevin 2013). At present, electrical energy is mostly used to drive potentiostats (Nevin et al. 2010; Jourdin et al. 2015; Marshall et al. 2013; Patil et al. 2015; Zhang et al. 2013) and maintain cathode potentials through direct or indirect electron transfer via H2 production. However, potentiostats are more expensive than high-power electrodes and are difficult to control in large electrodes; thus, the former is impractical for long-term operations at a large scale (Rosenbaum et al. 2011).
Few studies (Giddings et al. 2015; Xiang et al. 2017) have demonstrated that DC power can drive electron uptake at a cathode in MES. However, several factors restrict the performance of MES with a DC power supply. Microbial catalytic activity may be affected by voltage, electrode spacing, catholyte composition, and other factors. Microbial community can change over time during long-term operation. Limited efforts have also been devoted to investigating factors influencing the performance of MES with a DC power supply. Therefore, an MES system with DC power as an energy supply was examined in this study. Influential factors, such as voltage, electrode spacing, and catholyte composition, were investigated. The performance of MES and the changes in microbial community under a long-term operation with the DC power were also evaluated. This study could provide helpful insights into the practical operation of MES in large-scale systems in order to improve the efficiency and economic value of MES.
Materials and methods
Source of microorganisms and medium
Planktonic cells from an MES system designed by Song et al. (2018) were collected, centrifuged, suspended in fresh catholyte, and cultivated in a hydrogen-containing syngas mixture [H2/CO2 (80/20, v/v)]. Subsequent rapid culture transfers (5% inoculum dose) were performed every 3 days. The rapid transfers were used to ensure that the microbial community in MES was stable and the dominant product was acetate. This mixed microbial culture was used as a microbial inoculum for MES. The inoculum in the cathode was derived from the same microbial inoculum batch in each factor experiment.
A mineral synthetic medium was used as the catholyte (per liter of deionized water) containing 50 mL of PETC salt solution, which was originally used as culture medium for the strain Clostridium ljungdahlii PETCT, and named after the strain PETC (Tanner et al. 1993), 10 mL of trace element solutions, 0.5 g of Cys-HCl, 10 mL of Wolfe’s vitamin solution, and 1 g of NaHCO3. The pH of this medium was adjusted to 7. The anodic medium (per liter of deionized water) was composed of 50 mL L−1 PETC salt solution, 6 g L−1 NaCl, and 2 g L−1 KCl. The PETC salt solution consisted of the following (per liter of deionized water): 1 g of NH4Cl, 0.1 g of KCl, 0.2 g of MgSO4·7H2O, 0.8 g of NaCl, 0.1 g of KH2PO4, and 0.02 g of CaCl2. The trace element solution contained the following (per liter of deionized water): 2 g of nitrilotriacetic acid, 1.3 g of MnCl2·4H2O, 0.4 g of FeSO4·7H2O, 0.2 g of CoCl2·2H2O, 0.2 g of ZnSO4·7H2O, 0.02 g of CuCl2·2H2O, 0.02 g of NiCl2·6H2O, 0.02 g of Na2MoO4·2H2O, 0.02 g of Na2SeO3, and 0.025 g of Na2WO4·2H2O. Wolfe’s vitamin solution was made of the following (per liter of deionized water): 2.0 mg of biotin, 2.0 mg of folic acid, 10.0 mg of pyridoxine hydrochloride, 5.0 mg of thiamine·HCl, 5.0 mg of riboflavin, 5.0 mg of nicotinic acid, 5.0 mg of calcium d-(+)-pantothenate, 0.1 mg of vitamin B12, 5.0 mg of p-aminobenzoic acid, and 5.0 mg of thioctic acid.
MES experiment
All experiments were conducted in double-chamber H-type glass reactors with an effective volume of 280 mL in anode and cathode compartments. Such compartments were separated by a proton exchange membrane (PEM, Nafion 117, Dupont Co., USA). A 5 cm × 2.5 cm dimensionally stable anode (DSA, Baoji Longsheng Nonferrous Metal Co., Ltd., China) was used as anode, and a 5 cm × 5 cm carbon felt (Hunan Jiuhua Carbon High-tech Co., Ltd., China) was utilized as a cathode.
After assembly and sterilization, the corresponding medium was added to the cathode and anode chambers. The mixed culture from 5% (V/V) inoculate was anoxically placed into the cathode chamber for all the MES systems in this study. The anode chamber was continually gassed with N2. A magnetic stir plate was installed into the cathode to ensure homogeneous mixing, and 100% CO2 was continually gassed (3 mL min−1) into the cathode chamber. Voltage with a power supply (IT6720, Itech, China) was applied to the circuit by connecting the positive pole and negative pole of a power supply to the anode and the cathode, respectively. An external resistor (10 Ω) was connected to the negative pole of the power supply and the cathode. Current generation was monitored using a precision multimeter with a data acquisition system (2700, Keithley, USA). No supplemental nutrients were used in the experiments. All the reactors were kept at room temperature (25 ± 2 °C).
Analysis methods
Salinity and conductivity were measured with a conductivity meter (DDSJ-308F, Leici, China). Organic acids were measured using a high-performance liquid chromatography apparatus (1260, Agilent Technologies, USA) (Nevin et al. 2010). Electrochemical impedance spectroscopy (EIS) was performed through a potentiostat (CHI660D, Shanghai Chen Hua Instrument Co., Ltd., China). The cathode, anode, and Ag/AgCl were used as working electrode, counter electrode, and reference electrodes, respectively. The frequency range of the EIS was 0.01–100 kHz. The Coulombic efficiency (CE) was calculated as \({\text{CE}} = C_{\text{P}} /C_{\text{T}} \times 100\% ,\) where CT is the total Coulomb consumption calculated by integrating the area under the current–time curve (i–t curve). CP is the Coulomb in the product, and its calculation formula is CP = b × n×F, where b is the number of electrons in the product (8 eq mol−1), n is the number of moles of the product, and F is Faraday constant (96,485 C mol−1). The volumetric acetate production rate = acetate concentration/number of days of reaction. The highest volumetric acetate production rate was obtained by intercomparison.
Microbial community
Samples were collected from planktonic cells in the MES at 16, 31, 46, 61, 76, and 91 days. Total genomic DNA was extracted from the samples by using a PowerSoil DNA isolation kit (MO BIO Laboratories Inc., USA) in accordance with the manufacturer’s protocol. DNA quality was assessed at a ratio of 260 nm/280 nm through a NanoDrop spectrophotometer (ND-2000, NanoDrop Technologies Inc., USA), and a highly pure genomic DNA (A260/A280 = 1.8) was used for Illumina high-throughput sequencing by GENEWIZ (Suzhou, China).
Results and discussion
Effect of the cell voltage
The production rate of acetate was optimized on the basis of a reaction time of 14 days. The MES performance was evaluated at different cell voltages. When the applied cell voltages were 1.5 and 2.0 V, no acetate was produced (Fig. 1a) and the currents of both experimental groups were close to zero. These results implied that the electrochemical reaction of carbon dioxide did not occur at these voltages. Giddings et al. (2015) suggested that MES cannot maintain a stable cathode potential when an applied cell voltage was lower than 3.0 V. However, in the present study, the acetate started to accumulate at an applied cell voltage of 2.5 V. It might be due to the higher synergistic catalytic activity of mixed culture than that of pure bacterium. The acetate production rates increased, as the applied cell voltage increased within a certain range. At an applied cell voltage of 3.0 V, the maximum acetate titer was 2.18 g L−1 with a volumetric acetate production rate of 0.156 g L−1 day−1. As the applied cell voltage increased to 3.5 and 4.0 V, the maximum acetate titer further increased to 2.91 and 3.37 g L−1, and the volumetric acetate production rate reached 0.208 and 0.248 g L−1 day−1, respectively. The maximum acetate titer and volumetric acetate production rate no longer increased as the voltage increased to 4.5 V. By contrast, the maximum acetate titer and the volumetric acetate production rate at 4.5 V were nearly the same as those at 4.0 V. In addition to acetate titer and production rate, the current of the MES system was significantly influenced by the applied cell voltage (Fig. 1b). When the applied cell voltages were 2.5 and 3.0 V, the currents were maintained at 4.2 and 8.7 mA, and did not fluctuate throughout the entire process. As the voltage further increased, the current increased and reached the maximum value of 30.8 mA (4.5 V). After 4 days, the current continuously declined until the end of the experiment. All the currents demonstrated the same trend when an applied voltage was larger than 3.0 V. This pattern was consistent with that of OD600 of MES (> 3.0 V) (Fig. 1c). A strong inhibition effect likely occurred at the later stage of MES when the applied voltage was above 3.0 V. However, the exact mechanism by which autotrophic microorganisms cause this phenomenon remains unknown. CE was further calculated (Fig. 1d). When the applied voltages were 2.5 and 3.0 V, CEs arrived at 60.9% and 65.5%, and these values were close to the obtained CE in a potentiostat-poised system (Bajracharya et al. 2015; Patil et al. 2015; Song et al. 2017). As the voltage further increased, CE decreased to 38.7% (3.5 V), 31.2% (4.0 V), and 31.9% (4.5 V). This result was different from that reported by Giddings et al. (2015), who showed that CE at high voltages (3.5 and 5.0 V) remained unchanged. They reported an MES system by using a pure bacterium, namely Sporomusa ovata, as a biocatalyst. S. ovata is an acetogen that adopts the Wood–Ljungdahl pathway to fix CO2 with the reducing power provided by H2. At high applied cell voltages, S. ovata mainly uses H2, which is abiotically produced at a cathode as an electron donor, rather than through direct electron transfer from the cathode to the microorganism (Giddings et al. 2015). With the high turnover of S. ovata for CO2 and H2, the latter can be efficiently consumed. Thus, the enhanced hydrogen production rate with the increased applied voltage nearly had no effect on CE. In our system, the mixed culture was used as a biocatalyst, and comprehensive synergistic actions might occur between different bacteria. In addition to acetogens, H2-producing strains and electrochemically active bacteria account for a large proportion of mixed cultures (Dong et al. 2018; Song et al. 2017, 2018). In the present study, a large amount of H2 was generated in the MES system with high electron consumption as the applied voltage increased. However, the supply of hydrogen might exceed the amount of hydrogen that could be consumed by acetogens during the chemical production processes in our MES system, resulting in a decrease in CE. After considering the CE and acetate yields of the MES system, 3.0 V was used as the optimum applied cell voltage for subsequent experiments.
Effect of spacing between electrodes
The electrode spacing of 8, 13, and 18 cm corresponded to the nearest part from the PEM, the middle part in the chamber, and the farthest part from the PEM, respectively. The highest volumetric acetate production rate (0.208 g L−1 day−1) was detected in the MES system with the 8-cm electrode spacing followed by the 13-cm spacing (0.156 g L−1 day−1) (Fig. 2a). This rate was 1.44- and 1.08-fold of that in the 18-cm spacing (0.144 g L−1 day−1). The volumetric acetate production rate dramatically increased as the electrode spacing narrowed. This result reflected the importance of electrode spacing to the acetate production rate of an MES system and was likely attributed to a decrease in ohmic losses when electrode spacing is narrowed (Cheng et al. 2006). Narrow spacing led to a low resistance in the MES system and a further remarkable increase in the current and volumetric acetate production rate. With current as a function of time (Fig. 2b), the highest current (14 mA) was observed in the MES system with 8-cm electrode spacing followed by 13 cm (10 mA). By contrast, the lowest current (9.5 mA) was found in the MES system with 18-cm electrode spacing. Cyclic voltammetry values at three different electrode spacings were further determined (Fig. 2c). MES with 8-cm electrode spacing exhibited a significant reductive peak. The reductive peak current was 0.036 A at − 0.24 V potential, and this value was higher than that with 13-cm electrode spacing (0.024 A). No significant reductive peak was observed in the MES system with the electrode spacing of 18 cm. The high reduction peak current in the MES with 8-cm electrode spacing is attributed to a decrease in solution resistance.
Effect of PETC salt solution
The performance of MES with S. ovata as a biocatalyst in potentiostat-poised systems is affected by the composition of a medium (Ammam et al. 2016). In this research, four different PETC salt solutions at a fixed voltage (3.0 V) and a fixed spacing (13 cm spacing) were chosen to evaluate their effects on the MES performance and to study the effect of PETC salt solution in catholyte. In Fig. 3a, the volumetric acetate production rate increased when the PETC concentration increased from 0.5× to 2×. A higher PETC concentration (4×) did not further enhance the acetate production. The maximum volumetric acetate production rate in the MES system with 2 × PETC concentration was 11.8% higher than that of the MES with standard PETC (1×). The current was also significantly influenced by the PETC concentration (Fig. 3b). The current increased with the PETC concentration increased, and the highest current was 14 mA (4× PETC). The salinity and conductivity at different PETC concentrations were further determined (Table 1). Salinity ranged from 1.73 to 8.41 g L−1, and conductivity increased from 3.45 to 16.83 mS cm−1 as the PETC concentration increased from 0.5× to 4×. The result showed that the increase in PETC concentration could increase medium conductivity and salinity. An increase in medium conductivity reduces internal resistance and improves electrochemical reaction (Fan et al. 2008), but an increase in salinity may inhibit microbial growth. This phenomenon was observed in the change in OD600 (Fig. 3c). In particular, OD600 of MES (0.5× PETC) was the lowest, and OD600 of MES significantly increased with standard PETC (1×). When the PETC concentration further increased to 2×, OD600 decreased slightly at the later stage of the reaction, which could partially explain the 11% increase in acetate production at different salinity gradients. OD600 significantly decreased at 4× PETC and led to a reduction in acetate production. This phenomenon was similar to previous reports (Li et al. 2018), which demonstrated that the growth of S. ovata is inhibited when the buffer concentration is further increased to 200 mM. These results demonstrated that high PETC concentration did not significantly improve the acetate production rate in MES.
Effect of trace elements
Trace elements can be used as cofactors for enzymes involved in microbial metabolic pathways, and these elements can affect microbial activity in MES. Six different trace element solutions at a fixed voltage (3.0 V), a fixed spacing (13 cm spacing), and a standard PETC (1×) were selected to test their effects on the MES performance. In Fig. 4a, the maximum acetate titer increased by 1.4-fold from 1.99 to 2.82 g L−1 as the trace elements increased from 0.5× to 8×. The maximum volumetric acetate production rate in the MES system with 8× trace elements was 25.4% higher than that of the MES with standard trace elements (1×). When the trace elements further increased to 12×, the maximum acetate titer decreased to 2.48 g L−1. The current (Fig. 4b) in MES with 0.5× trace elements was the lowest (7.3 mA), and the current slowly increased from 9.2 to 11.1 mA as the trace elements increased from 1× to 8×. The current further increased to 13.8 mA when the trace elements increased to 12×. Salinity ranged from 2.64 to 3.15 g L−1, and conductivity increased from 5.21 to 6.29 mS cm−1 as the trace element concentration increased from 0.5× to 12× (Table 1). The OD diagram in Fig. 4c shows that the OD600 of MES significantly increased with 4× and 8× trace elements, and the corresponding acetate concentration rapidly increased. OD600 significantly decreased at 12× trace elements, leading to a decrease in the acetate production. The changes in the conductivity and salinity caused by trace elements were subtle compared with those associated with the PETC salt solution. This result implied that the addition of trace elements could improve the microbial activity of MES performance. The highest current was obtained from 12× trace elements because their conductivity was the highest among other elements. However, high salt concentrations inhibited microbial growth, leading to acetate decrease. These results demonstrated the feasibility of enhancing the rate of MES by appropriately increasing the trace element contents.
Long-term operation of MES for a DC power system
The MES system was operated for 100 days at a fixed voltage (3.0 V), a fixed spacing (8 cm), PETC (2×), and trace elements (8×) to further investigate the effects of long-term operation on the MES system. In Fig. 5a, the acetate titer continued to increase over time, and the acetate titer was 3.5 g L−1 on day 14. The maximum volumetric acetate production rate obtained during this period was 0.25 g L−1 day−1. Afterward, the acetate titer continuously increased and reached its maximum (7.8 g L−1) on day 62. However, the volumetric acetate production rate slightly decreased to 0.125 g L−1 day−1. The acetate concentration then began to decline gradually until day 100, and the acetate titer decreased to 5.3 g L−1 at the end of the experiment. The current fluctuated around 9 mA for 35 days (Fig. 5b). On day 42, the current tendency started to increase continuously. After the current remained steady for 18 days, it decreased gradually until the end of the reaction. The results showed that the acetate production rate continued to increase during the early stage of the reaction, and this increase slowed down at the middle stage. At the late stage, instead of accumulating, the acetate titer decreased until the end of the experiment. Similar to other reports (Bajracharya et al. 2015; Zhang et al. 2013), this phenomenon might be due to the thickening of the biofilm and the inhibitory effect of high acetate concentrations on the mixed cultures.
The planktonic cells in the cathode chamber from the MES reactor were sampled periodically to analyze the microbial community. In Fig. 6a, on day 16, the most abundant phylum was Proteobacteria followed by Firmicutes and Bacteroidetes. Later, the abundance of Proteobacteria first declined and then increased again. Its abundance reached 88.4% on day 91. Conversely, Firmicutes exhibited an opposite trend; that is, its abundance first increased and reached its maximum (55.9%) on day 46 and decreased continuously thereafter. In comparison with the changes in Firmicutes and Proteobacteria, the change in Bacteroidetes was minimal, and its abundance remained at 3–7.3% throughout the experiment.
Bacterial communities were also identified at the genus level (Fig. 6b). The predominant genera in the microbial community in the inoculum included Acetobacterium, Enterobacteriaceae, Arcobacter, Sulfurospirillum, and Thioclava. Acetobacterium can produce acetate from CO2 and H2 (Blaser et al. 2013). In the present study, Acetobacterium abundance increased over time, reached the maximum (52.9%) on day 46, and continuously decreased until the end of the experiment. This abundance trend of Acetobacterium might be used to explain the fluctuation of acetate production in the entire experimental period. In particular, the increased abundance of Acetobacterium was beneficial to acetate production at the early stage of the reaction. A high acetate concentration might inhibit the enrichment of Acetobacterium, resulting in the decline of acetate. Arcobacter is an electrochemically active bacterium responsible for electron transfer via an electrode (Fedorovich et al. 2009). The abundance of Arcobacter increased from 8.6 to 19% over time and stabilized until the end of the experiment, indicating that the electrochemically active bacterium was slowly enriched in the MES. Sulfurospirillum, which is microaerophilic (Marshall et al. 2017), was 8.5% abundant on day 16, and then, its abundance declined over time. Sulfurospirillum consumed the trace amounts of oxygen that diffused from the anode, maintaining an anaerobic environment for the rest of the community. However, the oxygen generation at the anode slowed down because of the decrease in the current in MES. Therefore, the abundance of Sulfurospirillum decreased. On the contrary, the abundance of Thioclava which is a sulfur-oxidizing bacterium (Sorokin et al. 2005) increased over time. Enterobacteriaceae is a facultative anaerobe that ferments sugars to produce lactic acid and various other end products (Song et al. 2017). In the present study, the abundance of Enterobacteriaceae decreased as the acetate concentration increased. After 61 days, the abundance of Enterobacteriaceae increased again. In summary, the abundance of Sulfurospirillum and Thioclava continuously decreased and increased, respectively. The abundance of Arcobacter increased and stabilized, while that of Acetobacterium increased and then decreased. By contrast, the abundance of Enterobacteriaceae decreased and then increased. The variation in Acetobacterium, which is an important bacterial genus in the MES system and has also been reported in other studies (Marshall et al. 2013; Patil et al. 2015), was consistent with that of the acetate titer in the solution. High acetate concentrations can inhibit the abundance of Acetobacterium over time. Thus, several separation methods, such as ion-exchange resin (Bajracharya et al. 2017) and liquid membrane extraction (Batlle-Vilanova et al. 2017), should be applied to improve the acetate production rate in the reaction.
Practical significance and perspectives
This work investigated and optimized various factors, including voltages, electrode distance, and catholyte composition, in the proposed MES system with a DC power supply. Current density, volumetric production rate, maximum acetate titer, and CE were calculated and compared with those described in other reported MES systems (Table 2). In MES systems with a potentiostat, carbon felt is the most commonly used cathode material (Bajracharya et al. 2015; Patil et al. 2015; Song et al. 2017). Strategies that aim to improve MES performance have been focusing on increasing the specific surface area or accelerating the electron transfer rate in cathodes, such as graphite granules (Marshall et al. 2013), reticulated vitreous carbon (Jourdin et al. 2014, 2015), and rGO-carbon felt (Song et al. 2017). In the present study, a relatively high volumetric acetate production rate (0.25 g L−1 day−1) was obtained. This rate was higher than most other MES systems but lower than the MES systems with graphite granules (Marshall et al. 2013). The relatively high acetate concentration can also be obtained (7.8 g L−1), which is less than the current highest acetate concentration of 11 g L−1 (Jourdin et al. 2015). However, the reported highest acetate production rate and acetate concentration were both obtained with the potentiostat. Compared with other MES systems with a DC power supply (Giddings et al. 2015; Xiang et al. 2017), our acetate production rate is the highest.
These results implied that the relatively high volumetric acetate production rate could be obtained in MES with a DC power supply. Compared with the modification of electrode materials, peripheral limitations, such as voltage, electrode spacing, and electrolyte composition, could be optimized to enhance the MES performance. In the present study, the regulation of voltage, electrode spacing, and trace elements in catholyte could significantly improve the MES performance, whereas the contribution of the regulation of PETC salt solution in catholytes to its improvement was negligible. Economic cost was a key challenge for the practical operation of MES in large-scale systems. Thus, shortening the spacing between electrodes could be an efficient and economical strategy compared with increasing the voltage and concentration of electrolyte compositions. With the structural limitation of the double-chamber reactor in the experiment, 8 cm was the closest electrode spacing in the conducted operation. The distance of the electrodes can be further decreased in a plate frame reactor (Gildemyn et al. 2015). However, the effect of oxygen penetration by water electrolysis in the anode should also be considered when reducing the spacing between electrodes. The acetate production rate decreased in later reactions, and the volumetric acetate production of 0.25 g L−1 day−1 on day 14 decreased to 0.125 g L−1 day−1 on day 62 because acetate accumulation affected the microbial community. Therefore, future studies should focus on new reactor structures to reduce internal resistance, increase CO2 mass transport, and maintain a high acetate production rate by solving the inhibition of high product concentrations.
Conclusions
The results show that a high concentration of acetate can be obtained using MES with a DC power source. The optimization studies have shown that MES performance mainly depends on the applied cell voltage, electrode spacing, and trace elements in the catholyte. The acetate production rate declined at the late stage of our long-term MES operation because of the change in microbial community due to the accumulation of acetate. These results indicate that MES with DC power sources can achieve a relatively high volumetric acetate production rate by factors optimization. This study provides valuable information for the construction and operation of more efficient and economical MES in large-scale systems.
Availability of data and materials
All data generated or analyzed during this study are included in this article
Abbreviations
- MES:
-
microbial electrosynthesis
- CE:
-
Coulombic efficiency
- CV:
-
cyclic voltammograms
References
Ammam F, Tremblay PL, Lizak DM, Zhang T (2016) Effect of tungstate on acetate and ethanol production by the electrosynthetic bacterium Sporomusa ovata. Biotechnol Biofuels 9:163
Bajracharya S, ter Heijne A, Benetton XD, Vanbroekhoven K, Buisman CJN, Strik DPBTB, Pant D (2015) Carbon dioxide reduction by mixed and pure cultures in microbial electrosynthesis using an assembly of graphite felt and stainless steel as a cathode. Bioresour Technol 195:14–24
Bajracharya S, Burga BVD, Vanbroekhovena K, Wever HD, Buisman CJN, Panta D, Strik DPBTB (2017) In situ acetate separation in microbial electrosynthesis from CO2 using ion-exchange resin. Electrochim Acta 237:267–275
Batlle-Vilanova P, Ganigué R, Ramió-Pujol S, Bañera L, Jiménez G, Hidalgo M, Balaguer MD, Colprima J, Puiga S (2017) Microbial electrosynthesis of butyrate from carbon dioxide: production and extraction. Bioelectrochemistry 117:57–64
Blaser MB, Dreisbach LK, Conrad R (2013) Carbon isotope fractionation of 11 acetogenic strains grown on H2 and CO2. Appl Environ Microb 79(6):1787–1794
Cheng SA, Liu H, Logan BE (2006) Increased power generation in a continuous flow MFC with advective flow through the porous anode and reduced electrode spacing. Environ Sci Technol 40:2426–2432
Christodoulou X, Velasquez-Orta SB (2016) Microbial electrosynthesis and anaerobic fermentation: an economic evaluation for acetic acid production from CO2 and CO. Environ Sci Technol 50:11234–11242
Dong ZW, Wang HQ, Tian SH, Yang Y, Yuan H, Huang Q, Song TS, Xie JJ (2018) Fluidized granular activated carbon electrode for efficient microbial electrosynthesis of acetate from carbon dioxide. Bioresour Technol 269:203–209
Fan YZ, Sharbrough E, Liu H (2008) Quantification of the internal resistance distribution of microbial fuel cells. Environ Sci Technol 42:8101–8107
Fedorovich V, Knighton MC, Pagaling E, Ward FB, Free A, Goryanin I (2009) Novel electrochemically active bacterium phylogenetically related to arcobacter butzleri, isolated from a microbial fuel cell. Appl Environ Microb 75(23):7326–7334
Giddings CGS, Nevin KP, Woodward T, Lovley DR, Butler CS (2015) Simplifying microbial electrosynthesis reactor design. Front Microbiol 6:468
Gildemyn S, Verbeeck K, Slabbinck R, Andersen SJ, Prevoteau A, Rabaey K (2015) Integrated production, extraction, and concentration of acetic acid from CO2 through microbial electrosynthesis. Environ Sci Tech Lett 2(11):325–328
Gildemyn S, Verbeeck K, Jansen R, Rabaey K (2017) The type of ion selective membrane determines stability and production levels of microbial electrosynthesis. Bioresour Technol 224:358–364
Jourdin L, Freguia S, Donose BC, Chen J, Wallace GG, Keller J, Flexer V (2014) A novel carbon nanotube modified scaffold as an efficient biocathode material for improved microbial electrosynthesis. J Mater Chem A 2(32):13093–13102
Jourdin L, Grieger T, Monetti J, Flexer V, Freguia S, Lu Y, Chen J, Romano M, Wallace GG, Keller J (2015) High acetic acid production rate obtained by microbial electrosynthesis from carbon dioxide. Environ Sci Technol 49(22):13566–13574
Li XH, Angelidaki I, Zhang YF (2018) Salinity-gradient energy driven microbial electrosynthesis of value-added chemicals from CO2 reduction. Water Res 142:396–404
Lovley DR (2011) Live wires: direct extracellular electron exchange for bioenergy and the bioremediation of energy-related contamination. Energy Environ Sci 4(12):4896–4906
Lovley DR, Nevin KP (2013) Electrobiocommodities: powering microbial production of fuels and commodity chemicals from carbon dioxide with electricity. Curr Opin Biotechnol 24(3):385–390
Marshall CW, Ross DE, Fichot EB, Norman RS, May HD (2013) Long-term operation of microbial electrosynthesis systems improves acetate production by autotrophic microbiomes. Environ Sci Technol 47(11):6023–6029
Marshall CW, Ross DE, Handley KM, Weisenhorn PB, Edirisinghe JN, Henry CS, Gilbert JA, May HD, Norman RS (2017) Metabolic reconstruction and modeling microbial electrosynthesis. Sci Rep 7:8391
Modestra JA, Mohan SK (2017) Microbial electrosynthesis of carboxylic acids through CO2 reduction with selectively enriched biocatalyst: microbial dynamics. J CO2 Utiliz 20:190–199
Nevin KP, Woodard TL, Franks AE, Summers ZM, Lovley DR (2010) Microbial electrosynthesis: feeding microbes electricity to convert carbon dioxide and water to multicarbon extracellular organic compounds. MBio 1(2):e00103-10
Nevin KP, Hensley SA, Franks AE, Summers ZM, Ou JH, Woodard TL, Snoeyenbos-West OL, Lovley DR (2011) Electrosynthesis of organic compounds from carbon dioxide is catalyzed by a diversity of acetogenic microorganisms. Appl Environ Microb 77(9):2882–2886
Nie HR, Zhang T, Cui MM, Lu HY, Lovley DR, Russell TP (2013) Improved cathode for high efficient microbial-catalyzed reduction in microbial electrosynthesis cells. Phys Chem Chem Phys 15(34):14290–14294
O’Neill BC, Oppenheimer M (2002) Dangerous climate impacts and the Kyoto protocol. Science 296:1971–1972
Patil SA, Arends JBA, Vanwonterghem I, van Meerbergen J, Guo K, Tyson GW, Rabaey K (2015) Selective enrichment establishes a stable performing community for microbial electrosynthesis of acetate from CO2. Environ Sci Technol 49(14):8833–8843
Rabaey K, Rozendal RA (2010) Microbial electrosynthesis revisiting the electrical route for microbial production. Nat Rev Microbiol 8:706–716
Rosenbaum M, Aulenta F, Villano M, Angenent LT (2011) Cathodes as electron donors for microbial metabolism: which extracellular electron transfer mechanisms are involved? Bioresour Technol 102:324–333
Shin HJ, Jung KA, Nam CW, Park JM (2017) A genetic approach for microbial electrosynthesis system as biocommodities production platform. Bioresour Technol 245:1421–1429
Song TS, Zhang HK, Liu HX, Zhang DL, Wang HQ, Yang Y, Yuan H, Xie JJ (2017) High efficiency microbial electrosynthesis of acetate from carbon dioxide by a self-assembled electroactive biofilm. Bioresour Technol 243:573–582
Song TS, Fei KQ, Zhang HK, Yuan H, Yang Y, Ouyang PK, Xie JJ (2018) High efficiency microbial electrosynthesis of acetate from carbon dioxide using a novel graphene–nickel foam as cathode. J Chem Technol Biotechnol 93:457–466
Sorokin DY, Tourova TP, Spiridonova EM, Rainey FA, Muyzer G (2005) Thioclava pacifica gen. nov., sp. nov., a novel facultatively autotrophic, marine, sulfur-oxidizing bacterium from a near-shore sulfidic hydrothermal area. Int J Syst Evol Microbiol 55:1069–1075
Tanner RS, Miller LM, Yang D (1993) Clostridium ljungdahlii sp. nov., an acetogenic species in clostridial rRNA homology group I. Int J Syst Bacteriol 43:232–236
Xiang YB, Liu GL, Zhang RD, Lu YB, Luo HP (2017) High-efficient acetate production from carbon dioxide using a bioanode microbial electrosynthesis system with bipolar membrane. Bioresour Technol 233:227–235
Zhang T, Nie HR, Bain TS, Lu HY, Cui MM, Snoeyenbos-West OL, Franks AE, Nevin KP, Russell TP, Lovley DR (2013) Improved cathode materials for microbial electrosynthesis. Energy Environ Sci 6(1):217–224
Acknowledgements
Not applicable.
Funding
This work was supported by the National Key Research and Development Program of China (2018YFA0901300); the National Natural Science Foundation of China (Grant No.: 21878150); the Major projects of natural science research in Jiangsu Province (Grant No.: 15KJA530002); the Key projects of modern agriculture in Jiangsu Province (Grant No.: BE2018394); Fund from the State Key Laboratory of Materials-Oriented Chemical Engineering (ZK201605); and the Jiangsu Synergetic Innovation Center for Advanced Bio-Manufacture.
Author information
Authors and Affiliations
Contributions
TS built the experimental design and contributed to writing of the manuscript. GR performed the MES experiment and scientific monitoring. HQ performed the microbial community analysis. QH performed the electrochemical analysis, the LC, and electrochemical analysis. JJ contributed to the discussion and writing of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Song, Ts., Wang, G., Wang, H. et al. Experimental evaluation of the influential factors of acetate production driven by a DC power system via CO2 reduction through microbial electrosynthesis. Bioresour. Bioprocess. 6, 29 (2019). https://doi.org/10.1186/s40643-019-0265-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40643-019-0265-5